 Ketek has had a rocky history. To begin with, the FDA was slow in approving the drug due to fraudulent clinical trials. In 2004, it was approved to treat everything from bronchitis to sinusitis, but mainly based on 5 years of sales in Europe. Just months after its approval in the US, Ketek claimed its first victim: a patient died from liver failure after taking the antibiotic for a mild respiratory tract infection.
Ketek has had a rocky history. To begin with, the FDA was slow in approving the drug due to fraudulent clinical trials. In 2004, it was approved to treat everything from bronchitis to sinusitis, but mainly based on 5 years of sales in Europe. Just months after its approval in the US, Ketek claimed its first victim: a patient died from liver failure after taking the antibiotic for a mild respiratory tract infection.The FDA was accused of allowing fraudulent data to be used in the approval process and a criminal investigation, along with Senate hearings followed.
By the end of 2006 a panel of FDA advisers recommended severe restrictions on Ketek. The FDA removed two of its approved uses (bacterial sinusitis and chronic bronchitis) and slapped a black box warning on the drug.
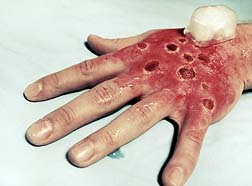
In early 2007, a Canadian woman was diagnosed with toxic epidermal necrolysis (TEN), a more severe stage of SJS, after taking Ketek. Allegedly, Sanofi-Aventis was "considering" a change on its label that would warn potential consumers and physicians of other side effects such as SJS and TEN that causes hundreds of deaths each year.
READ MORE SJS KETEK LEGAL NEWS
Mainly as a result of the Ketek mess, the FDA section that handles antibiotics "is one of, if not the most, difficult, environments to work in at the agency right now," according to Patrick Ronan, a former FDA chief of staff who now consults for health care companies.
