Zimmer NexGen CR-Flex KNEE REPLACEMENT
Since the Zimmer NexGen knee replacement system has been on the market, almost half a million people in the US alone have had Zimmer knee implants. However, the Zimmer knee replacement, namely the NexGen CR-Flex Porous Femoral component, has recently been linked to a variety of problems, from loosening of the implant to failure of the replacement knee, requiring revision surgery. As a result, some medical professionals have called for a Zimmer NexGen CR-Flex recall of this component, though that has not yet happened. Zimmer NexGen lawsuits are now being reviewed by Zimmer knee attorneys for people who have experienced problems with the Zimmer NexGen CR-Flex Porous Femoral component. Some patients have also experienced problems with the Zimmer Durom Cup hip replacement.
FREE ZIMMER NEXGEN LAWSUIT EVALUATION
GET LEGAL HELP NOW
Zimmer NexGen CR-Flex Porous Femoral Component
 Zimmer NexGen Knee was approved by the FDA in 1994, and since that time more than three million NexGen knees have been implanted worldwide. But data now indicates that certain Zimmer knees--namely the NexGen CR-Flex Porous Femoral component--have been associated with unexplained pain after knee replacement, loosening of the implant and failure of the replacement knee leading to revision surgery.
Zimmer NexGen Knee was approved by the FDA in 1994, and since that time more than three million NexGen knees have been implanted worldwide. But data now indicates that certain Zimmer knees--namely the NexGen CR-Flex Porous Femoral component--have been associated with unexplained pain after knee replacement, loosening of the implant and failure of the replacement knee leading to revision surgery.Zimmer NexGen CR-Flex Porous Femoral knee component failure rates are high--according to a study published in the American Academy of Orthopedic Surgeons. This study was also noted in Zimmer's SEC Form 8-k filing, Exhibit 99.1 (3/11/10), with Zimmer's response:
"Zimmer's NexGen ® CR-Flex Porous Femoral component was the subject of a study of 108 patients ("The High Failure Rate of a High-Flex Total Knee Arthroplasty Design") by Drs. Berger and Della Valle of Rush University Medical Center. According to the published abstract, Drs. Berger and Della Valle revised 9 (8.3%) patients for femoral loosening and pain. The study also reported that 39 (36%) patients showed evidence of radiographic loosening and that "[l]oosening and revision were not related to surgeon, approach or patient type."
"The NexGen CR-Flex Porous component has a strong track record of clinical success. According to the 2009 Australian National Joint Replacement Registry report, Zimmer's NexGen cementless total knee arthroplasty (TKA) components recorded the lowest revision rate (reported as revisions per 100 observed component years) of all major cementless TKA systems utilized in the market (0.5 revisions per 100 observed component years). Of the 7,100 implantations using the NexGen CR Porous or NexGen CR-Flex Porous systems since 2004, revisions were reported for 120 patients, representing a revision rate of 1.7%, inclusive of all components and reasons for revision.
"The performance of the NexGen CR-Flex Porous Femoral component in the Australian National Joint Registry and in Zimmer's post-market surveillance activities demonstrates that it is a safe and effective product when used as indicated in the surgical technique. More than 150,000 NexGen CR and NexGen CR-Flex Porous Femoral components have been sold since 2003. Sales of the NexGen CR-Flex Porous component represented approximately 2% of the company's knee revenues in 2009."
The design of the NexGen CR-Flex knee has a high-flex porous femoral component that attaches to the bottom of the thighbone instead of using cement to keep the knee replacements in place. Some orthopedic surgeons believe this design to be defective and unreasonably dangerous, and can potentially expose patients to an unnecessary risk of problems.
Bone Scan to Diagnose Knee Replacement Problems
A bone scan involves injecting a harmless amount of radioactive material (radiotracer) into a vein, which travels through the bloodstream to the bones and organs. As the substance wears away, it gives off radiation. Then this radiation is detected by a camera that takes an image of the bone surrounding the artificial knee. Usually the scan will take about one hour.
Zimmer NexGen Timeline
 2002: Zimmer announced " Positive Results of Minimally Invasive Total Hip Replacement Study and Plans for a Dedicated Surgeon Training Institute". Dr Richard Berger, M.D, a consultant for Zimmer, was then pioneering a type of small-incision surgery that allowed patients to leave the hospital on the day of surgery and he was prominently featured in a press release about Zimmer's plans to build the training facility.
2002: Zimmer announced " Positive Results of Minimally Invasive Total Hip Replacement Study and Plans for a Dedicated Surgeon Training Institute". Dr Richard Berger, M.D, a consultant for Zimmer, was then pioneering a type of small-incision surgery that allowed patients to leave the hospital on the day of surgery and he was prominently featured in a press release about Zimmer's plans to build the training facility.2005: Dr Berger had implanted the NexGen CR-Flex device—which is supposed to last about 15 years—to about 125 patients.
Early 2006: Some X-rays from patients who had received the device in 2005 showed lines where the implant met the thigh bone, an indication that the device was loose and had not fused completely.
2007: Dr. Berger, although still a Zimmer consultant, had stopped using the NexGen knee replacement and said that several other surgeons had also experienced problems with it.
March 2010: The failure rate data (above) was presented by a group of knee surgeons at a conference of the American Academy of Orthopedic Surgeons.
June 2010: The New York Times reported disputes between Zimmer and two of its top consultants. One of the disputes involved the NexGen CR-Flex knee component. The consultants claimed the components were defective and failed soon after they were implanted, but Zimmer officials blamed the surgeons' techniques for the failure.
One of the consultants was Dr. Berger. Over the past decade, he had received more than $8 million from Zimmer for his services and in 2005 he implanted Zimmer's NexGen CR-Flex device in about 125 patients, according to the NYT.
Berger noticed that some X-rays showed lines where the implant met the thigh bone, which indicated that the device had not fused completely. Although patients were able to walk, they experienced severe pain. Berger also found Zimmer's knee showed signs of loosening in about half of all patients and many needed to be replaced. Berger reported his findings to Zimmer.
Another consultant, Lawrence Dorr, M.D, had reported problems to Zimmer regarding its Durom cup hip component. Although the cup failed within a few years of being implanted, Zimmer claimed the problem was with Dorr's technique and not because the device was defective. (Durom cup lawsuits are pending.)
July 2010: Senator Charles Grassley (R-Iowa) issued a letter to Zimmer Holdings, which focused on the pharmaceutical company's transparency regarding its disclosure of payments to healthcare professionals. In the letter, Grassley refers to the June, 2010 article in The New York Times:
"I was troubled by last month's New York Times account of Zimmer's response to the allegations of safety concerns raised by two of its consultants. Specifically, The New York Times reported that one of the surgeons with whom Zimmer had a financial relationship, Dr. Richard Berger, raised concerns to the company a few years ago about the premature failure of a Zimmer knee, the NexGen CR-Flex.1 According to the article, Dr. Berger was a long-time consultant for Zimmer--a financial relationship that spanned more a decade--with Dr. Berger receiving more than $8 million during that time frame. The New York Times also reported that a second Zimmer consultant, Dr. Lawrence Dorr, alerted other doctors that Zimmer's Durom hip device was failing a few years after they were implanted in patients. According to The New York Times, the two doctors were not alone in their concerns about the device failures. In both cases, however, Zimmer responded that it was the surgeons' technique, not the devices that were flawed."
The letter asks Zimmer officials to disclose the way they track the long-term performance of orthopedic devices--their product assurance and complaint handling systems. Zimmer reportedly met with members of Senator Grassley's Committee staff and received notice of satisfactorily responding to the requests.
December 2010: While there has not been a recall of the Zimmer NexGen CR-Flex system or components, on December 2, 2010, the certain lots of the Zimmer NexGen LPS Flex Gender were recalled. The recall included the following Zimmer NexGen Complete Knee Solution LPS Components:
- Zimmer NexGen LPS Femoral Component, Size E Right, sterile, REF 00-5996-015-02
- Zimmer NexGen LPS Femoral Component, Size F Right, sterile, REF 00-5996-016-02
- Zimmer NexGen LPS Femoral Component, Size F Left, sterile, REF 00-5996-016-01
- Zimmer NexGen LPS Femoral Component, Size G Right, sterile, REF 00-5996-017-02
- Zimmer NexGen LPS Femoral Component, Size G Left, sterile, REF 00-5996-017-01
- Zimmer NexGen LPS Flex Gender Femoral Component, Size E Right, sterile, REF 00-5764-015-52
- Zimmer NexGen LPS Flex Gender Femoral Component, Size E Left, sterile, REF 00-5764-015-51
- Zimmer NexGen LPS Flex Gender Femoral Component, Size F Right, sterile, REF 00-5764-016-52
- Zimmer NexGen LPS Flex Gender Femoral Component, Size G Left, sterile, REF 00-5764-017-51
The NexGen LPS knee replacement components involved in the recall were due to these components having a nonconforming internal CAM radius (nonconforming geometry). According to the recall notice, surgeons were notified, but no action was to be taken by surgeons.
Zimmer NexGen Knee Replacement Legal Help
If you or a loved one has suffered damages in this case, please click the link below and your complaint will be sent to a lawyer who may evaluate your claim at no cost or obligation.
Last updated on
ZIMMER NEXGEN LEGAL ARTICLES AND INTERVIEWS
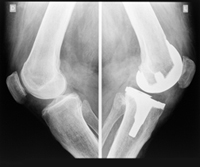
Better Off before Zimmer NexGen Knee
Zimmer NexGen Knee Patient Wanted Her Leg Amputated
Huntsville, AL: Pam had a Zimmer NexGen knee replacement two years ago, and she has been suffering ever since.
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Randy
on
Tom Williamson
on
Tuck
on
Philip G. Buckeridge
on
Arizona
on
I met with my surgeon and his response was I need more PT which I did. After going back to the surgeon a couples times with the same complaint and not getting any good response, I chose to get a second opinion. My second opinion surgeon identified the device had become loose and was just flopping around.
The only thing to do was to remove the device and put in a different one. So I chose this route and a revision was done in 2011. Because of the damage from the original device the surgeon didn’t have much to work with once he got inside the knee. He explained prior to the surgery that this might be the case. So he had to do a few other things to make sure this device would stay in place.
I did all the PT that’s required but my range of motion was never back to normal, I walk with a limp, and I am now if chronic pain 24/7 due to a nerve being compromised during the revision surgery ( I don’t fault the surgeon because this is one of the risks that can happen with this type of surgery.)
My quality of life has gone out the window all because of the original device loosening. I cannot walk very far because it’s too painful being on my feet. I use a power wheelchair and cannot be away from home for very long because my leg requires elevation most of the time.
Wisconsin
on
California
on
Anonymous
on
Ohio
on
Wisconsin
on
South Carolina
on
Arizona
on
Please contact me and let me know if this is from a defective knee.
It is the Zimmer CR-Flex
Texas
on
Texas
on
Texas
on
New York
on
Arizona
on
Wisconsin
on