Posts Tagged ‘ Seroquel ’
5 of the Highest Settlement Awards for 2010
December 29th, 2010. By Kristine B
 Once again we find ourselves at the end of a year—and it’s been a pretty big year for lawsuits. So, in celebration of the year that was, we’ve compiled some of the biggest settlements—in terms of amount of the award or settlement—of 2010. My deepest thanks to the incredibly talented, wonderful, smart, beautiful, fantastic duo of Lucy C. and Abi K. for helping me compile this list…
Once again we find ourselves at the end of a year—and it’s been a pretty big year for lawsuits. So, in celebration of the year that was, we’ve compiled some of the biggest settlements—in terms of amount of the award or settlement—of 2010. My deepest thanks to the incredibly talented, wonderful, smart, beautiful, fantastic duo of Lucy C. and Abi K. for helping me compile this list…
Personal Injury Lawsuits
When it came to personal injury, wrongful death and negligence lawsuits, there were some pretty large awards and settlements given out in 2010.
One settlement of $176 million was approved in a lawsuit filed after a 2003 nightclub fire in Rhode Island killed 100 people. The settlement money will go to survivors of the fire who suffered serious injury and to the children who lost parents in the fire.
Meanwhile, in one of the largest jury awards ever in the US, a jury in El Paso awarded $132 million to victims of a bus crash in which two people were killed and several others were injured. The vehicle reportedly crashed when the driver began speeding and eating at the same time. The bus rolled down an embankment.
Defective Products
Speaking of automobiles, a settlement was reached earlier this year between Volkswagen of America, Inc. and vehicle owners who said their cars leaked during rainstorms. The defective product lawsuit alleged certain Volkswagen and Audi models contained design defects that allowed water to enter the passenger component and, in some cases, damaged electronic components of the vehicle. As part of the settlement, Audi was required to pay $10,000 to each of the class representatives, $9.2 million in fees and $675,000 in costs for the class-counsel firms.
And in another defective product lawsuit, Medtronic agreed to pay $268 million to settle lawsuits regarding the company’s Sprint Fidelis Leads. The leads were alleged to have been defectively designed, allowing the wires to crack and causing unnecessary shocks to patients’ hearts.
And, in a defective drugs lawsuit, the maker of Seroquel agreed to pay 17,500 patients a total of $198 million to settle allegations that the medication caused illnesses such as diabetes.
Big Pharma Lawsuits – Who Got Hit with the Biggest Settlement?
October 25th, 2010. By LucyC
 It seems that every month practically, one pharmaceutical company or another makes the news for bending rules around marketing. Mis-marketing, which could also be called consumer fraud, can result in serious, if not life-changing consequences for people making decisions about their health.
It seems that every month practically, one pharmaceutical company or another makes the news for bending rules around marketing. Mis-marketing, which could also be called consumer fraud, can result in serious, if not life-changing consequences for people making decisions about their health.
Recently, I came across a list of the largest settlements paid by 11 pharmaceutical companies for bending the rules. The fines total a staggering $6 billion. The more frequent offender, according to the company that compiled the list, is Eli Lilly. They paid more than $1.4 billion in fines all for various violations for just one drug—Zyprexa.
And then there’s Pfizer, who paid $2.3 billion for ‘mis-marketing’ a number of drugs including Bextra, Geodon, Lyrica and Zyvox.
These drugs are used to treat everything from schizophrenia to epilepsy to diabetes, and the consequences of not having the correct information may have resulted in serious adverse health events, possibly even death for some.
Not surprisingly, people tend to be very interested when the big boys get caught behaving badly, for a variety of reasons, not the least of which being that we feel our trust has been betrayed. We trust drug companies, and the medical profession in general, to give us the straight goods because it’s a matter of life and death. Why would you not be straight about that? Well, the answer is, not surprisingly, money. And lots of it. But eventually the offenders do get caught. And that leads to drug lawsuits, criminal investigations and ultimately, very large fines.
So, without further ado—here’s a list of the big offenders—who took them on, what for and how much they paid, with acknowledgement to FiercePharma.com who actually did the homework on this.
Novartis
With: U.S. Attorney’s office for the Eastern District of Pennsylvania
When: Sept. 30, 2010
Why: Novartis agreed to a $422.5 million settlement with the Eastern District of Pennsylvania for its off-label promotion of Trileptal and other allegations against Diovan, Exforge, Sandostatin, Tekturna and Zelnorm. (oh, and ps, Novartis is recruiting for a Senior Brand Manager for Prevacid…)
Forest Labs
With: Dept. of Justice
When: Sept. 15, 2010
Why: After marketing Levothroid, an unapproved thyroid drug, Forest Labs received a $313 million penalty. The settlement also covered Forest’s off-label use of Celexa for children’s use.
Allergan
With: Dept. of Justice
When: Sept. 1, 2010
Why: Allergan’s was fined $600 million by the Department of Justice. The settlement was broken into two parts: $375 million in fines and $225 milion in civil penalties, all of which stemmed from its off-label use of Botox for headaches, pain management and cerebral palsy.
Elan
With: U.S. Attorney’s Office in Massachusetts
When: July 15, 2010
Why: Elan received a $203.5 million fine for its marketing of Zonegran, an epilepsy drug.
Johnson & Johnson
With: Department of Justice
When: April 29, 2010
Why: Though J&J is most recently famous or a rash of phantom recalls, two of the troubled drugmaker’s subsidiaries received a $81 million penalty for off-label promotions of Topamax, an epilepsy drug.
AstraZeneca
With: U.S. Attorney’s office in Philadelphia
When: April 27, 2010
Why: In the same week as the J&J settlement, AstraZeneca was fined $520 million misleading doctors and patients about the safety of its antipsychotic drug Seroquel.
Abbott
With: Twenty-three states
When: Jan. 7, 2010
Why: In a case involving 23 different states, Abbott Laboratories and its partner, Fournier Industrie et Sante, were ordered to pay $22.5 million for blocking the states from obtaining a cheaper alternative for its cholesterol drug, TriCor. (btw, Abbott Labs is the one who brought you beetle parts in Similac, causing the recent Similac recall…)
Eli Lilly
With: Connecticut
When: Sept. 29, 2009
Why: A total of 13 states total had filed suit against Eli Lilly for Zyprexa marketing issues, but the company was ordered to pay $25 million to Connecticut in this ruling.
Eli Lilly
With: West Virginia Attorney General
When: August 21, 2009
Why: In another Zyprexa case, West Virginia Attorney General Darrell McGraw levied $2 billion in fines against Eli Lilly. In the end, the company agreed to $22.5 million in fines.
Merck
With: 35 states’ attorney offices
When: July 15, 2009
Why: Following a 35 state investigations into the Enhance study of Vytorin, Merck paid $5.4 million in fines, without admitting fault in the cases.
Sanofi-Aventis
With: Department of Justice
When: May 28, 2009
Why: In an agreement with the federal government, Sanofi paid $95.5 million total, to the federal government, state Medicaid agencies and other public health service agencies, all for its subsidiary Aventis’ nasal spray price inflation between 1995 and 2000.
GlaxoSmithKline
With: U.S. Attorney’s office in Colorado
When: Jan. 29, 2009
Why: After seven years of off-label promotion on nine of its best-selling drugs, GlaxoSmithKline (GSK) was ordered to pay $400 million to the U.S. Attorney’s office in Colorado.
Pfizer
With: Department of Justice
When: Jan. 26, 2009
Why: Right after acquiring Wyeth, Pfizer dropped a bombshell in its fourth quarter earnings report; the company was charged $2.3 billion for off-label promotions of its COX-2 drugs.
Eli Lilly
With: Department of Justice
When: Jan. 15, 2009
Why: In the first Zyprexa settlement (and one of three on our list), the Department of Justice levied $1.4 billion in fines against Eli Lilly. Also, as part of the settlement, the company pled guilty to a misdemeanor: violating the Food, Drug and Cosmetic Act.
Seroquel Settlements Continue…but What Drug is Next?
September 2nd, 2010. By LucyC
During the past few weeks we have seen AstraZeneca (AZ) settle some 17,500 Seroquel lawsuits—give or take. That puts them about two thirds of the way through a staggering 26,000 cases currently pending against them. So far the estimated amount of those settlements is around $198 million, according to media reports. That certainly isn’t chump change. But then the drug reportedly did about $4.9 billion in sales last year. You do the math.
According to a report on Bloomberg, AZ is still facing “at least 8,000 cases in both state and federal courts.” The cases allege that the drug, which was approved by the federal Food and Drug Administration for treatment of schizophrenia and mania associated with bipolar disorder, causes—or at least is associated with—the onset of diabetes. All the settlements so far have been negotiated out of court, so this week it was decided to give AZ a little more time to see if they could finish the job—settle all the outstanding cases—without having to go to court. We’ll see.
The average amount of the settlements per case is around $11,000. I wonder how much diabetes medication that buys?
But it seems that Seroquel isn’t the only drug associated with the development of adverse Read the rest of this entry »
Kevin Bacon Game helps Decipher Diabetes-related Drug Lawsuits
September 2nd, 2010. By AbiK
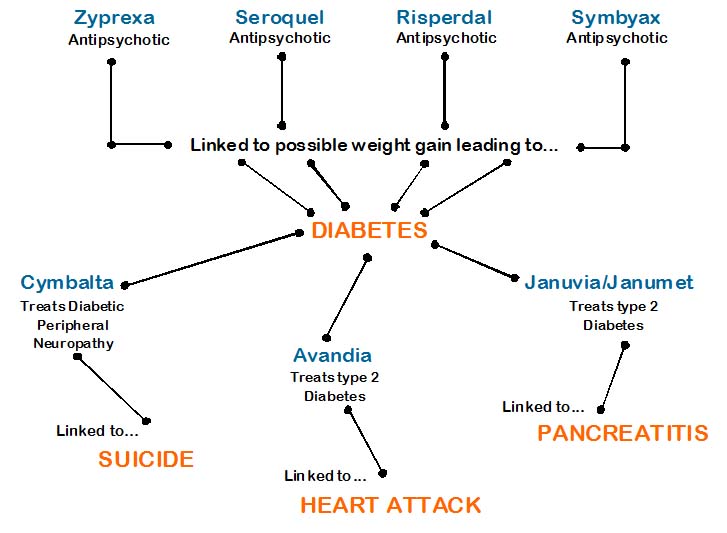
If you’ve ever wondered why certain drugs (Zyprexa, Avandia, Seroquel, Cymbalta…to name a few) seem to have something in common, but you just can never really put your finger on it, well, it’s time to look to the Kevin Bacon Game (aka, Six Degrees of Separation, with some liberties taken) to find out what those connections are…so here goes…
Seroquel in the US…Seroquel in the EU…
October 9th, 2009. By AbiK
 I was reading Jane’s post on drug companies’ unethical marketing tactics and thinking about Seroquel. As Jane pointed out (and as is reported over at bloomberg.com yesterday), Seroquel’s manufacturer, Astra Zeneca,
I was reading Jane’s post on drug companies’ unethical marketing tactics and thinking about Seroquel. As Jane pointed out (and as is reported over at bloomberg.com yesterday), Seroquel’s manufacturer, Astra Zeneca,
“advised its sales force to promote the antipsychotic drug Seroquel as “weight neutral” four years after company research found “clinically significant” weight gains in users, internal documents show.”
Now, given this revelation, and given Seroquel’s reported link to increased risk of diabetes, one might question what’s going on over in the EU where a panel recently gave AstraZeneca the green light for the prevention of major depression recurrence.
In a quote from the company (businessweek.com, 9/29/09),
“Following this new indication, Seroquel and Seroquel XR are the only agents approved in the European Union to treat all phases of bipolar disorder acute depressive episodes, acute manic episodes and maintenance treatment to prevent recurrence of any mood event in bipolar disorder.”
Oh, and in case you forgot, an application for the same (ie, to treat major depressive disorder) has been pending in the US since being filed in February, 2008.
Makes you wonder if the two continents across the pond from one another ever speak to one another…
Archive by Category
- Accidents (24)
- Airlines (9)
- Asbestos Mesothelioma (262)
- Automotive (25)
- Celebrity (14)
- Class Action (84)
- Complaints/Comments (15)
- Consumer Fraud (84)
- Contest (2)
- Court of Public Opinion (5)
- Crazy Sh*t Lawyers See (61)
- Criminal Law (4)
- Defective Products (111)
- DePuy ASR Hip Recall (2)
- Discrimination (22)
- Drugs/Medical (248)
- Elder Care Abuse (4)
- Emerging Issues (462)
- Employment (54)
- Environment (52)
- Financial (28)
- Food Illness (15)
- Human/Civil Rights (4)
- Insecurities (5)
- Insurance (16)
- Intellectual Property (16)
- Internet/E-commerce (19)
- lawsuits (161)
- Lawyers (20)
- Lawyers Giving Back (43)
- Lex Levity (10)
- Personal Injury (106)
- Pleading Ignorance (53)
- Real Estate (2)
- Recall (6)
- Scam (3)
- Securities (13)
- Settlement (81)
- Tort Reform (2)
- Totally Tortelicious (81)
- Veterans (11)
- Whistleblower (9)