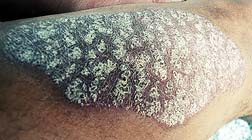
 Questions have been raised: Were the clinical trials adequate; did Genentech, the manufacturer, study the long-term effects of their drug and conduct adequate follow-up studies; and why did it take so long for the FDA to slap a black box warning on Raptiva?
Questions have been raised: Were the clinical trials adequate; did Genentech, the manufacturer, study the long-term effects of their drug and conduct adequate follow-up studies; and why did it take so long for the FDA to slap a black box warning on Raptiva?In an email statement, Tara Cooper, a spokeswoman for Genentech said it is "working diligently with the FDA to put the right plans in place that will help protect patient safety," and the company is "evaluating all possible approaches to address the risk of PML with Raptiva use, including a risk minimization plan."
Perhaps the right plan would be to shelve the product until it can be determined that Raptiva benefits for psoriasis patients outweigh risks.
European regulators already urged the removal of Raptiva, saying the drug's therapeutic benefits are "modest" and that the drug is "associated with other serious side effects," including Guillain-Barre and Miller- Fisher syndromes and encephalitis. "Prescribers should not issue any new prescriptions for Raptiva and should review the treatment of patients currently receiving the medicine to assess the most appropriate alternatives," European regulators said.
READ MORE RAPTIVA LEGAL NEWS
Just four months later, the FDA announced that it is reviewing new information and will take appropriate steps to ensure that the risks of Raptiva do not outweigh its benefits. Meanwhile, countless consumers are taking Rapitva: about 7.5 million Americans have psoriasis. The drug generates about $120 million a year in sales, mainly from sales in the US.
Many patients who have had adverse effects from Raptiva are now seeking legal help and considering lawsuits against Genentech.
