LAWSUITS NEWS & LEGAL INFORMATION
Raptiva Brain Infection Legal News Articles & Interviews
Raptiva Lawsuits Cause of Controversy
 January 10, 2013. By Heidi Turner.
January 10, 2013. By Heidi Turner.Raptiva Pulled from Market Due to Concerns About Side Effects
 December 17, 2012. By Heidi Turner.
December 17, 2012. By Heidi Turner.Why Raptiva was Withdrawn
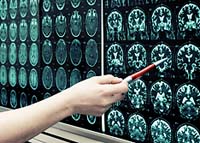 June 8, 2009. By Jane Mundy.
June 8, 2009. By Jane Mundy.Raptiva PML: Brains Appear Eaten Away
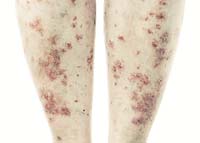 May 28, 2009. By Gordon Gibb.
May 28, 2009. By Gordon Gibb.Raptiva Cause for Concern
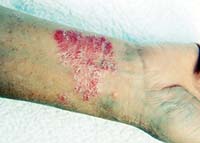 May 18, 2009. By Jane Mundy.
May 18, 2009. By Jane Mundy.Concerns after Raptiva Withdrawal
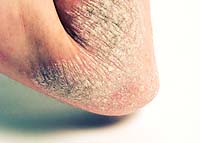 May 6, 2009. By Jane Mundy.
May 6, 2009. By Jane Mundy.The Real Impact of FDA Raptiva Removal
 April 27, 2009. By Gordon Gibb.
April 27, 2009. By Gordon Gibb.Raptiva Risks Outweighed Benefits
 April 14, 2009. By Jane Mundy.
April 14, 2009. By Jane Mundy.Raptiva Joins List of Drugs with Deadly Side Effects
 April 2, 2009. By Heidi Turner.
April 2, 2009. By Heidi Turner.Psoriasis Drug Raptiva Suspended in Other Countries. Not in US.
 March 26, 2009. By Gordon Gibb.
March 26, 2009. By Gordon Gibb.- Do Raptiva's Risks outweigh Benefits? By Jane Mundy (Mar-16-09)
- Raptiva Side Effects Include Meningitis By Jane Mundy (Mar-3-09)
- Raptiva: An Itch That Can Kill By Gordon Gibb (Feb-21-09)
