 To recap, in June 2012, the US Food and Drug Administration (FDA) sent a warning letter to Zithromax manufacturer Pfizer Inc. (Pfizer) over a brochure Pfizer had distributed espousing the virtues of Zmax, an extended-release version of Zithromax (azithromycin). The FDA, in its view, found the manufacturer lacking when it came to communicating side effects to consumers and, specifically, Zithromax Stevens Johnson Syndrome.
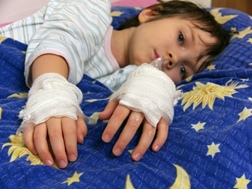
To recap, in June 2012, the US Food and Drug Administration (FDA) sent a warning letter to Zithromax manufacturer Pfizer Inc. (Pfizer) over a brochure Pfizer had distributed espousing the virtues of Zmax, an extended-release version of Zithromax (azithromycin). The FDA, in its view, found the manufacturer lacking when it came to communicating side effects to consumers and, specifically, Zithromax Stevens Johnson Syndrome.Stevens Johnson Syndrome, or SJS for short, is a horrific allergic reaction to Zithromax that begins with Zithromax rash, and can quickly expand to blisters and massive loss of upper dermis (the outer layer of the skin) similar in character to serious burns.
SJS and its more devious cousin, Toxic epidermal necrolysis (TEN), can and has been proven to be fatal. Those who survive the horrors of SJS and TEN can be scarred for life - both emotionally and physically.
No one knows what causes Stevens Johnson Syndrome, beyond an allergic reaction to medication that differs from one person to the next. Zithromax could trigger SJS in one individual, but not another. Similarly, an individual can use azithromycin successfully in the past, and yet develop an allergic reaction to it later on. There appears to be no pattern - and Zithromax is not the only suspected culprit: other medications can trigger similar adverse reactions.
What plaintiffs in many a Zithromax lawsuit object to is not knowing about the possibility. Plaintiffs in a Zithromax Stevens Johnson Syndrome lawsuit filed in 2012 made just that point, in court documents.
“[Zithromax] lacked sufficient warnings of the hazards and dangers to users of said product and failed to provide safeguards to prevent the injuries sustained by the plaintiff,” the Alexander Zithromax lawsuit said (Aleigh Alexander et al. v. Pfizer Inc. et al., Case No. 155812/2012, in the Supreme Court of the State of New York, County of New York).
In May of 2014, it appeared as if Pfizer was making detailed information available for doctors and the medical community, but was satisfied with glossing over the details for consumers, leaving them with information about possible Zithromax side effects in the most general of terms.
Some 20 months later, little appears to have changed. On the official Zithromax page on Pfizer.com, a single link for consumers references the potential for rare, but serious side effects that include severe allergic reactions and severe skin rash or blisters, among others. Caregivers are advised to take their children immediately to a hospital should this happen. Parents and caregivers are also invited to ask their doctor or medical health professional for a detailed Zithromax Professional package insert, which - presumably - is not packaged directly with the medication.
Stevens Johnson Syndrome is not identified or referenced within the information page intended for consumers. A web page, Zithromax.com, is also referenced but does not work. The consumer page for Zithromax on the Pfizer site is shown as having last been updated in June 2011.
The link for medical professionals, on the other hand, is much more detailed and has been updated more recently. In May of last year, the warnings and precautions section for hypersensitivity was updated thus: “Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) have been reported in patients on azithromycin therapy.
READ MORE ZITHROMAX SJS LEGAL NEWS
“If an allergic reaction occurs, the drug should be discontinued and appropriate therapy should be instituted. Physicians should be aware that allergic symptoms may reappear when symptomatic therapy has been discontinued.”
With more consumers mining the online portals for research and subject material, Pfizer has not provided new or updated information for almost five years for the exclusive use of consumers. Unless they are told by their prescribing doctor or other medical professional, consumers have no idea as to what Stevens Johnson Syndrome is, or that Zithromax - and many other medications for that matter - can trigger a horrific reaction that can cause dire health issues, and even death.
