 A citizen petition from June 2008 to the FDA raised concern over, in the petitioner's view, the risks posed by terbutaline to the fetus, neonate and mother—and that long-term use of terbutaline for the treatment of preterm labor is not efficacious.
A citizen petition from June 2008 to the FDA raised concern over, in the petitioner's view, the risks posed by terbutaline to the fetus, neonate and mother—and that long-term use of terbutaline for the treatment of preterm labor is not efficacious.Earlier this month the FDA issued a statement that it partially granted but at the same time denied various aspects of the petitioner's request. Beyond the classification change for pregnancy risk, the FDA noted that labeling for terbutaline needs to be revised to include the new safety information.
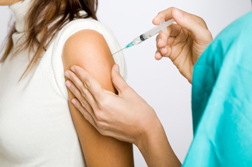
Terbutaline is a popular drug, administered by injection, designed to prevent pregnant women from prematurely giving birth. Various groups, such as the Triplet Connection, advocate for the drug as an important tool for women carrying twins and triplets. Women carrying multiples stand a higher risk of giving birth prematurely.
"It would be alarming to me to see it become unavailable," said Janet Bleyl, president and founder of the Triplet Connection, in comments published February 18 in USA Today. "It has made a life-or-death difference for many, many of our families."
However, concern with terbutaline remains, and has been under some scrutiny long before the citizen petition to the FDA in 2008.
In fact, it was in 1996 that the National Women's Health Network (NWHN) first petitioned the FDA to take action with regard to terbutaline, which carries rare but serious risks to the heart. As a result of the NWHN advocacy, the FDA issued a letter to doctors in 1997. "I hope the FDA's new warning will help us finish the job that got started with that letter," said Cynthia Pearson of the NWHN.
The FDA said 16 deaths among pregnant women have been reported to the agency between 2009 and 1976, when the drug first appeared on the market. It identified 12 cases of serious heart events reported to the agency between 1998 and July 2009.
The FDA "has concluded that the risk of serious adverse events outweighs any potential benefit to pregnant women" from prolonged treatment with terbutaline injections or from either short- or long-term treatment with the drug in pill form, the warning said.
READ MORE TERBUTALINE SULPHATE LEGAL NEWS
"There's no proof of effectiveness," he said.
Serious heart problems are rare, but the drug routinely triggers other side effects. Women sometimes feel jittery and have rapid heart rates and difficulty sleeping, said Thorp.
Women should not be given injections of the drug terbutaline for more than three days, says the FDA, "because of the potential for serious maternal heart problems and death." The agency is now requiring a boxed warning. USA Today reported that many women are given terbutaline for weeks at a time via a terbutaline pump. That practice may now change, although doctors have always had the moral and ethical right to prescribe an approved drug as they see fit, beyond the recommendation of the FDA.

READER COMMENTS
Elizabeth
on
Jim Reichmann
on