 The good news, if there is any, is that the recall does not affect any stents which have been successfully implanted, as the problem is not with the stent itself but the device used to implant it. Rest assured that patients receiving the above-noted stent who underwent the procedure without complication, have nothing to worry about.
The good news, if there is any, is that the recall does not affect any stents which have been successfully implanted, as the problem is not with the stent itself but the device used to implant it. Rest assured that patients receiving the above-noted stent who underwent the procedure without complication, have nothing to worry about.Curiously, however the recall was issued in early June. Only now, more than two months later, has the news begun to circulate.
According to information posted with the US Food and Drug Administration (FDA), the agency took delivery June 6th of a voluntary recall notice from Boston Scientific with regard to the NexStent delivery system, an entity that was approved by the FDA in 2006 for patients with compromised neck arteries—the vital pathway carrying blood to the brain.
The problem, according to the manufacturer and the FDA, is that the tip of the device used to place the stent can break off during implantation. Such an occurrence raises the possibility of a stroke, or internal injury. While there have been no reports of injury or complications resulting from the defect—or at least none were reported—Boston Scientific decided to alert doctors, hospitals and the medical community given that 2,200 of the devices have been distributed to hospitals worldwide from June 2007 to May of this year.
Given that problems with the nickel-titanium tubes only occur during placement, Boston Scientific is of the view that patients having already received a stent in a successful procedure are not at all, at risk. And to the uninitiated, a problem with an insertion device might be viewed as no big deal. Okay, so a piece breaks off. The surgeon can just reach in and grab the offending bit, right?
Not exactly, as patients familiar with the insertion procedure can attest.
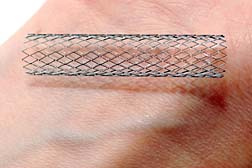
The insertion of a stent is necessary when an artery is in danger of closing, or is compromised for any reason. A stent is a short, mesh tube fashioned from surgical steel that is inserted into the artery at the problematic region and serves to hold the artery open, ensuring adequate blood flow.
However, unlike bypass surgery or similar major procedure, stent insertion is minimally invasive, which reduces the size of the wound and significantly improves recovery time. Specifically, a small incision is made in the groin and a catheter is inserted, and threaded up through the artery to the desired spot. Then a partially-collapsed, self-expanding stent is threaded up through the catheter to the insertion point, at which time the stent insertion device accomplishes the proper placement, the stent expands to its full dimensional compliment, out comes the insertion device and the catheter, and Bob's your uncle. The patient has a small incision in his, or her groin to show for it.
READ MORE NEXSTENT LEGAL NEWS
A much more invasive surgical procedure may be required to retrieve the broken tip.
The stent business is important to Boston Scientific, with sales of its drug-coated Taxus stent reported at $382 million in the most recent quarter. That makes the Taxus stent Boston Scientific's best-selling product.

READER COMMENTS
Roy Prater
on