LAWSUITS NEWS & LEGAL INFORMATION
Composix Kugel Mesh Patch
The Kugel Mesh patch was first recalled in 2005, after the FDA received reports that the Composix Kugel Mesh patch had caused many severe injuries. Two more Kugel Mesh recalls followed. Since that time, thousands of Kugel Mesh lawsuits have been filed against Davol, Inc. and its parent company Bard, and many more hernia patients with Composix Kugel Mesh may be entitled to file a Kugel Mesh lawsuit.
The US Food and Drug Administration first approved the Kugel Mesh Patch in 1996. The Composix Kugel Mesh patch is surgically implanted via a small incision, positioned behind the hernia, and its 'memory coil ring' opens the patch behind the herniated area. The mesh patch is then supposed to repair the hernia.
The memory ring is designed to aid in deployment of the patch, but it can break when increased stress is placed on it during certain surgical placement techniques. If the ring should break, the broken ends could poke through the mesh and create bowel perforations and/or a condition called "chronic enteric fistulae."
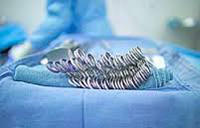
 The first Kugel Mesh recall occurred in 2005. It was recalled again in 2006 and 2007, both times to recall several other sizes of the device. The FDA, along with Davol, Inc., a subsidiary of C.R. Bard, Inc., recalled certain models of the Bard Composix Kugel Mesh Patch (below) after reports that the memory ring had broken, causing a number of painful and serious injuries in hernia patients.
The first Kugel Mesh recall occurred in 2005. It was recalled again in 2006 and 2007, both times to recall several other sizes of the device. The FDA, along with Davol, Inc., a subsidiary of C.R. Bard, Inc., recalled certain models of the Bard Composix Kugel Mesh Patch (below) after reports that the memory ring had broken, causing a number of painful and serious injuries in hernia patients.
Because the reports of side effects were so severe, the FDA designated specific lots of the Kugel Mesh X-Large Patch as a Class I recall. This level of recall is defined as a product that is dangerous or defective and could cause serious health problems or death.
According to the FDA Recall Notice, "Patients who have been implanted with a Composix Kugel Mesh Patch during hernia surgery should seek medical attention immediately if they experience symptoms that could be associated with ring breakage. These symptoms include the following:
The product codes for the Dec-05, Jan-06 and Mar-06 recalls are:
Kugel Mesh attorneys are also investigating the following hernia mesh patches manufactured by Davol and Bard that have not been recalled, but defects have been reported.
For further information on the three recalls, visit the FDA website.
In its second-quarter filings, C.R. Bard reported it settled some lawsuits related to use of the Composix Kugel Mesh Patch. According to The Providence Journal (07/06/11), Bard will pay $184 million to settle 2,600 of approximately 3,600 lawsuits filed against the company to date. The lawsuits claimed that the company's Composix Kugel Mesh hernia patch caused serious medical complications including bowel perforation, sepsis, intestinal fistulas and abdominal abscess formation.
In 2010 a jury in the U.S. District Court for the District of Rhode Island found Davol and Bard liable for injuries suffered by plaintiffs Christopher Thorpe and Laure Thorpe. The drug device company was also found liable for the failure to warn of dangers associated with the patch. The Thorpe case resulted in a $1.5 million verdict for the plaintiffs. Christopher Thorpe' attorneys claimed that he suffered severe internal injuries caused by a broken plastic ring on the hernia repair mesh, which included an abdominal wall abscess and fistula and resulted in him undergoing a number of surgeries to repair the damage. The jury award $1.3 million by the jury for personal injury damages and $200,000 for loss of consortium.
Thousands of cases are pending and underway against the Kugel Mesh manufacturer in Rhode Island federal court' first consolidated multidistrict litigation (MDL). Almost 2,000 more cases are pending in Rhode Island Superior Court.
By the time of the first recall in December 2005, Davol had sold about 32,000 of its Kugel Mesh Patches worldwide since 2002. In 2005 alone, the device sales reached $11 million.
Last updated on
En Español KUGEL MESH
FREE KUGEL MESH HERNIA PATCH LAWSUIT EVALUATION
Send your Kugel Mesh Hernia Patch claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Kugel Mesh Patch
The memory ring is designed to aid in deployment of the patch, but it can break when increased stress is placed on it during certain surgical placement techniques. If the ring should break, the broken ends could poke through the mesh and create bowel perforations and/or a condition called "chronic enteric fistulae."
Kugel Mesh Recalls
 The first Kugel Mesh recall occurred in 2005. It was recalled again in 2006 and 2007, both times to recall several other sizes of the device. The FDA, along with Davol, Inc., a subsidiary of C.R. Bard, Inc., recalled certain models of the Bard Composix Kugel Mesh Patch (below) after reports that the memory ring had broken, causing a number of painful and serious injuries in hernia patients.
The first Kugel Mesh recall occurred in 2005. It was recalled again in 2006 and 2007, both times to recall several other sizes of the device. The FDA, along with Davol, Inc., a subsidiary of C.R. Bard, Inc., recalled certain models of the Bard Composix Kugel Mesh Patch (below) after reports that the memory ring had broken, causing a number of painful and serious injuries in hernia patients.
Because the reports of side effects were so severe, the FDA designated specific lots of the Kugel Mesh X-Large Patch as a Class I recall. This level of recall is defined as a product that is dangerous or defective and could cause serious health problems or death.
According to the FDA Recall Notice, "Patients who have been implanted with a Composix Kugel Mesh Patch during hernia surgery should seek medical attention immediately if they experience symptoms that could be associated with ring breakage. These symptoms include the following:
- unexplained or persistent abdominal pain
- fever
- tenderness at the surgery site or other unusual symptoms
The product codes for the Dec-05, Jan-06 and Mar-06 recalls are:
| PC#0010206 | Bard Composix Kugel | Extra Large Oval | 8.7" x 10.7" |
| PC#0010207 | Bard Composix Kugel | Extra Large Oval | 10.8" x 13.7" |
| PC#0010208 | Bard Composix Kugel | Extra Large Oval | 7.7" x 9.7" |
| PC#0010209 | Bard Composix Kugel | Oval | 6.3" x 12.3" |
| PC#0010202 | Bard Composix Kugel Large | Oval | 5.4" x 7" |
| PC#0010204 | Bard Composix Kugel | Large Circle | 4.5" |
Kugel Mesh attorneys are also investigating the following hernia mesh patches manufactured by Davol and Bard that have not been recalled, but defects have been reported.
| 0010203 | Bard ® Composix ® Kugel | Small Circle | 7.6 cm |
| 0010201 | Bard ® Composix ® Kugel | Small Oval | 8 cm x 12 cm |
| 0010205 | Bard ® Composix ® Kugel | Medium Oval | 11 cm x 14 cm |
| Bard® Kugel® Hernia Patch | All sizes | ||
| Bard® VentraleX® Hernia Patch | All sizes | ||
| Bard® Modified Kugel™ Patch | All sizes | ||
| Bard® Composix® E/X Mesh | All sizes |
For further information on the three recalls, visit the FDA website.
Kugel Mesh Lawsuits
In 2010 a jury in the U.S. District Court for the District of Rhode Island found Davol and Bard liable for injuries suffered by plaintiffs Christopher Thorpe and Laure Thorpe. The drug device company was also found liable for the failure to warn of dangers associated with the patch. The Thorpe case resulted in a $1.5 million verdict for the plaintiffs. Christopher Thorpe' attorneys claimed that he suffered severe internal injuries caused by a broken plastic ring on the hernia repair mesh, which included an abdominal wall abscess and fistula and resulted in him undergoing a number of surgeries to repair the damage. The jury award $1.3 million by the jury for personal injury damages and $200,000 for loss of consortium.
Thousands of cases are pending and underway against the Kugel Mesh manufacturer in Rhode Island federal court' first consolidated multidistrict litigation (MDL). Almost 2,000 more cases are pending in Rhode Island Superior Court.
By the time of the first recall in December 2005, Davol had sold about 32,000 of its Kugel Mesh Patches worldwide since 2002. In 2005 alone, the device sales reached $11 million.
Kugel Mesh Legal Help
If you or a loved one has suffered bowel perforation or chronic enteric fistulae due to the Kugel Mesh patch, please send your complaint to a Kugel Mesh attorney by clicking the link below. Your claim will be reviewed by a Kugel Mesh lawyer at no cost.Last updated on
KUGEL MESH HERNIA PATCH LEGAL ARTICLES AND INTERVIEWS
Kugel Mesh One Helluva Mess
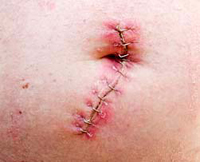
Living with Hernia versus Kugel Mesh
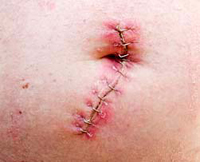
Kugel Mesh Victim Says She Would Rather Live with a Hernia

February 28, 2015
“If I knew that some Kugel Mesh had been recalled, I would have insisted on having it taken out sooner,” says Ann. “This mesh had 10 years to seriously damage my body.” READ MORE
Living with Hernia versus Kugel Mesh

December 14, 2014
Harry wishes he had spent more time researching Kugel Mesh before having surgery to treat a hernia. After two more unsuccessful surgeries to remove the Composix Kugel Mesh patch, Harry is filing a Kugel Mesh lawsuit. READ MORE
Kugel Mesh Victim Says She Would Rather Live with a Hernia

October 23, 2014
Mary wishes she never had a Kugel Mesh patch implanted to treat a hernia. In fact, she would rather live with a hernia than the Composix Kugel Mesh patch. READ MORE
READ MORE Surgical Mesh Settlements and Legal News
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Amy B.
on
Jeanne Loch
on
Robert walker
on
Sharon Rice
on
Bruce Sponholz
on
THERESA JUDD
on
Mark A. Beal
on
Shelly Roth
on
Claudius Richards
on
GSavage
on
Margot Ybarra
on
Alabama
on
Louisiana
on
Missouri
on
Wisconsin
on
Wisconsin
on
California
on
Pennsylvania
on
Colorado
on
Alabama
on
Maryland
on
Michigan
on
Texas
on
Iowa
on
California
on
I had a ventral hernia repaired with one of these patches. Is it possible that you can have complications with these things this long after surgery? I am concerned now.
What is the recommendation concerning the implant and now recall of this patch?
If I have this thing inside of me and it can break or already has broken how can they tell?
Kansas
on
California
on
This is not normal. I have sought out a new doctor and now have to have the mesh removed. This is a miserable surgery that takes many months to recover. I have no choice but to have it removed. The pain will not go away.
California
on
Washington
on
Texas
on
Idaho
on
Arizona
on