LAWSUITS NEWS & LEGAL INFORMATION
Januvia Pancreatic Cancer, Januvia Thyroid Cancer
Read our Januvia FAQ
Were you looking for Actos or Byetta or Diabetes Medication Lawsuits or Victoza Side Effects lawsuits?
By Jane Mundy
Januvia is a drug prescribed to help treat adults with type-2 diabetes. In September 25, 2009 the FDA issued a revision to the warning label of the drug Januvia or Janumet--a combination drug comprised of Januvia and Metformin--regarding Januvia side effects, including Januvia pancreatic cancer and Januvia thyroid cancer. If you have taken the Januvia diabetes drug and are concerned about Januvia and cancer and/or Januvia and pancreatitis, you should speak with an attorney about a possible Januvia lawsuit.
 Januvia is a type 2 diabetes that is also available in a combination pill (known as Janumet) that contains the drug metformin. (Janumet 50/500 is 500mg of Metformin plus 50mg of Januvia.)
Januvia is a type 2 diabetes that is also available in a combination pill (known as Janumet) that contains the drug metformin. (Janumet 50/500 is 500mg of Metformin plus 50mg of Januvia.)
Januvia (sitagliptin) and Janumet (sitagliptin/metformin) are the first in a new class of oral diabetes medications, called dipeptidyl peptidase-4 (DPP-4) inhibitors that improve blood sugar control in patients with type 2 diabetes. Januvia - which works by affecting different cells in the pancreas - first came on the market in late 2006. However, after that release, the FDA received dozens of reports of pancreatitis from patients who were using Januvia. For many, their symptoms were later resolved once they stopped taking the drug.
Januvia was approved by the FDA in October 2006. Between October 16, 2006 and February 9, 2009, the agency received 88 post-marketing cases of acute pancreatitis, including two cases of hemorrhagic or necrotizing pancreatitis. Sixty-six of those patients required hospitalization, and four of the patients were transferred to the intensive care unit. The FDA review of these 88 patients found that 19 patients (21 percent) of pancreatitis occurred within 30 days of starting Januvia. As well, just over half of the cases were resolved once Januvia was discontinued.
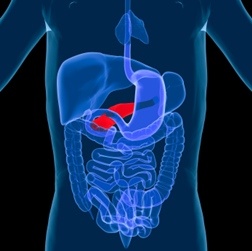 The FDA has asked Merck to update its Januvia warning label to reflect these new dangers and include:
The FDA has asked Merck to update its Januvia warning label to reflect these new dangers and include:
A more recent study (May 2009) found that Januvia could lead to a form of low-grade pancreatitis in some patients and a higher risk of pancreatic cancer in people who use the drug over a long period of time. Although Januvia has successfully lowered blood sugar in people with type 2 diabetes, health professionals are urging caution, particularly because the effect of the drug may not be evident for years. (It took more than 3 years to associate Byetta with pancreatitis and warnings have only recently been made public about Januvia, even though the FDA received adverse Januvia side effects from 2006.)
(It took more than 3 years to associate Byetta with pancreatitis and warnings have only recently been made public about Januvia, even though the FDA received adverse Januvia side effects from 2006.)
Meanwhile, a study published in 2011 in the journal Gastroenterology (07/11) found a link between the use of Januvia and an increased risk of pancreatitis and pancreatic cancer. According to researchers, patients who took Januvia had six times the increased risk of pancreatitis and 2.7 times the risk of pancreatic cancer. Other researchers, however, said the study was flawed and the drugs had no such increased risk.
Diabetes affects more than 23 million in the US alone and about 90-95 percent of those affected have type 2 diabetes. Diabetes is the fifth leading cause of death by disease in the US and costs approximately $132 billion per year in direct and indirect medical expenses. Furthermore, 85 percent of type 2 diabetes patients are overweight and 55 percent are considered obese.
Currently Januvia attorneys are investigating potential lawsuits against Merck & Co alleging product liability, negligence and failure to warn claims.
Last updated on
En Español JANUVIA
FREE JANUVIA LAWSUIT EVALUATION
Send your Januvia claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Januvia Pancreatic Cancer
 Januvia is a type 2 diabetes that is also available in a combination pill (known as Janumet) that contains the drug metformin. (Janumet 50/500 is 500mg of Metformin plus 50mg of Januvia.)
Januvia is a type 2 diabetes that is also available in a combination pill (known as Janumet) that contains the drug metformin. (Janumet 50/500 is 500mg of Metformin plus 50mg of Januvia.)
Januvia (sitagliptin) and Janumet (sitagliptin/metformin) are the first in a new class of oral diabetes medications, called dipeptidyl peptidase-4 (DPP-4) inhibitors that improve blood sugar control in patients with type 2 diabetes. Januvia - which works by affecting different cells in the pancreas - first came on the market in late 2006. However, after that release, the FDA received dozens of reports of pancreatitis from patients who were using Januvia. For many, their symptoms were later resolved once they stopped taking the drug.
Januvia/Janumet and Pancreatic Cancer
 The FDA has asked Merck to update its Januvia warning label to reflect these new dangers and include:
The FDA has asked Merck to update its Januvia warning label to reflect these new dangers and include:
- Information regarding post-marketing reports of acute pancreatitis, including the severe forms, hemorrhagic or necrotizing pancreatitis.
- Recommending that healthcare professionals monitor patients carefully for the development of pancreatitis after initiation or dose increases of sitagliptin or sitagliptin/metformin, and to discontinue sitagliptin or sitagliptin/metformin if pancreatitis is suspected while using these products.
- Information noting that sitagliptin has not been studied in patients with a history of pancreatitis. Therefore, it is not known whether these patients are at an increased risk for developing pancreatitis while using sitagliptin or sitagliptin/metformin.
- Sitagliptin or sitagliptin/metformin should be used with caution and with appropriate monitoring in patients with a history of pancreatitis.
A more recent study (May 2009) found that Januvia could lead to a form of low-grade pancreatitis in some patients and a higher risk of pancreatic cancer in people who use the drug over a long period of time. Although Januvia has successfully lowered blood sugar in people with type 2 diabetes, health professionals are urging caution, particularly because the effect of the drug may not be evident for years.
 (It took more than 3 years to associate Byetta with pancreatitis and warnings have only recently been made public about Januvia, even though the FDA received adverse Januvia side effects from 2006.)
(It took more than 3 years to associate Byetta with pancreatitis and warnings have only recently been made public about Januvia, even though the FDA received adverse Januvia side effects from 2006.)
Meanwhile, a study published in 2011 in the journal Gastroenterology (07/11) found a link between the use of Januvia and an increased risk of pancreatitis and pancreatic cancer. According to researchers, patients who took Januvia had six times the increased risk of pancreatitis and 2.7 times the risk of pancreatic cancer. Other researchers, however, said the study was flawed and the drugs had no such increased risk.
Diabetes affects more than 23 million in the US alone and about 90-95 percent of those affected have type 2 diabetes. Diabetes is the fifth leading cause of death by disease in the US and costs approximately $132 billion per year in direct and indirect medical expenses. Furthermore, 85 percent of type 2 diabetes patients are overweight and 55 percent are considered obese.
Currently Januvia attorneys are investigating potential lawsuits against Merck & Co alleging product liability, negligence and failure to warn claims.
Januvia Side Effects Legal Help
If you or a loved one has suffered damages in this case, please click the link below and your complaint will be sent to a lawyer who may evaluate your claim at no cost or obligation.Last updated on
JANUVIA LEGAL ARTICLES AND INTERVIEWS
Invokana Lawsuits Consolidated into MDL in New Jersey

Janumet Patient Felled by Cancer of the Pancreas in 2012

FDA Black Box Warning Adds Fuel to Invokana Lawsuits

Philadelphia, PA Final results from two independent clinical trials indicate the amputation risk for users of the Type 2 Diabetes drug Canagliflozin, marketed as Invokana, Invokamet, Invokamet XR, are much greater than previously understood.
The FDA was so concerned about the information the studies provided that it moved immediately to ensure consumers are warned about the increased risk of leg and foot amputations through a Boxed Warning label on all Invokana prescriptions.
“I think the fact that the independent clinical trials lead to a situation where the FDA went straight to a black box warning, its highest level of scrutiny, just shows how important and how critical this information is to the public,” says Brian J. McCormick Jr., a Philadelphia attorney from the firm of Ross Feller Casey.
To McCormick, Jr. the studies confirm and add significant weight to the many personal injury claims he and his firm are bringing forward on behalf of diabetic Americans that allege they have been injured as a result of using Invokana to control their Type 2 Diabetes Mellitus.
The studies (CANVAS Canagliflozin Cardiovascular Assessment Study) and the CANVAS-R (A Study of the Effects of Canagliflozin on Renal Endpoints in Adult Participants with Type 2 Diabetes Mellitus) showed, according to a FDA website safety warning (May 2017), that leg and foot amputation occurred about twice as often in patients treated with Canagliflozin compared to patients taking a placebo.
“It is a terrible progression for some of these patients,” says McCormick. “They go in and they have a diabetic foot ulcer and they don’t how bad it is. They have one amputation and then they go back and have another toe removed – and next thing they know they have half their foot removed.”
According to the FDA safety communication on May 15, 2017, “Amputations of the toe and middle of the foot were the most common; however, amputations involving the leg, below and above the knee, also occurred. Some patients had more than one amputation, some involving both limbs.”
“Our firm is investigating both Ketoacidosis cases and we are also investigating amputation cases,” says McCormick. “We have a number of clients we are working with and I think at the end of the day what this new information shows, and will show in court, is that Johnson & Johnson did know that this risk existed and chose not to do anything about it.”
Canagliflozin is licensed for sale in North America by Johnson & Johnson subsidiary Janssen Pharmaceuticals. The drug is designed to be used in combination with diet and exercise to lower blood sugar levels in Type 2 diabetes patients. The drug is a sodium-glucose cotransporter-2 (SGLT2) inhibitor that causes sugar to be eliminated through the urine.
Invokana was first approved for sale by the FDA in March 2015. Within three months the FDA had 100 reports of Ketoacidosis and kidney damage in Invokana users. In May 2015, the FDA ordered the label contain aKetoacidosis warning. By 2016, the FDA had added possible kidney damage to the Invokana warnings. In May 2017, it added increased risk of amputation.
“Some of these patients are otherwise healthy but for this terrible injury all of a sudden. They have been on this drug for a few months, or perhaps a few years and now they are seeing this Box Warning and putting two and two together,” McCormick says. “All of a sudden this is happening. No one told them about this and it would have changed how they handled their diabetes.”
The Invokana lawsuits have been consolidated in an MDL in New Jersey. The cases are going through case management process with discovery expected at a later date. It will be some time before the cases go to trial.
READ MORE

November 20, 2017
Trenton, NJ: The FDA warns of fracture risk with Invokana and Invokamet and more recently of a higher risk for foot and leg amputation. The lawsuit chickens have now come home to roost. READ MORE
Janumet Patient Felled by Cancer of the Pancreas in 2012

August 22, 2017
Los Angeles, CA: When Janumet (sitagliptin and metformin combined) was approved by the US Food and Drug Administration (FDA) in 2007, the type 2 diabetes drug was within five years of achieving sales of $1.7 Billion. That year, when Janumet hit blockbuster status by 2012, the parent drug of Janumet – Januvia – earned $4 Billion for manufacturer Merck & Co. Januvia has been associated with Januvia side effects, and has resulted in Januvia cancer lawsuits. READ MORE
FDA Black Box Warning Adds Fuel to Invokana Lawsuits

June 24, 2017
Philadelphia, PA Final results from two independent clinical trials indicate the amputation risk for users of the Type 2 Diabetes drug Canagliflozin, marketed as Invokana, Invokamet, Invokamet XR, are much greater than previously understood.
The FDA was so concerned about the information the studies provided that it moved immediately to ensure consumers are warned about the increased risk of leg and foot amputations through a Boxed Warning label on all Invokana prescriptions.
“I think the fact that the independent clinical trials lead to a situation where the FDA went straight to a black box warning, its highest level of scrutiny, just shows how important and how critical this information is to the public,” says Brian J. McCormick Jr., a Philadelphia attorney from the firm of Ross Feller Casey.
To McCormick, Jr. the studies confirm and add significant weight to the many personal injury claims he and his firm are bringing forward on behalf of diabetic Americans that allege they have been injured as a result of using Invokana to control their Type 2 Diabetes Mellitus.
The studies (CANVAS Canagliflozin Cardiovascular Assessment Study) and the CANVAS-R (A Study of the Effects of Canagliflozin on Renal Endpoints in Adult Participants with Type 2 Diabetes Mellitus) showed, according to a FDA website safety warning (May 2017), that leg and foot amputation occurred about twice as often in patients treated with Canagliflozin compared to patients taking a placebo.
“It is a terrible progression for some of these patients,” says McCormick. “They go in and they have a diabetic foot ulcer and they don’t how bad it is. They have one amputation and then they go back and have another toe removed – and next thing they know they have half their foot removed.”
According to the FDA safety communication on May 15, 2017, “Amputations of the toe and middle of the foot were the most common; however, amputations involving the leg, below and above the knee, also occurred. Some patients had more than one amputation, some involving both limbs.”
“Our firm is investigating both Ketoacidosis cases and we are also investigating amputation cases,” says McCormick. “We have a number of clients we are working with and I think at the end of the day what this new information shows, and will show in court, is that Johnson & Johnson did know that this risk existed and chose not to do anything about it.”
Canagliflozin is licensed for sale in North America by Johnson & Johnson subsidiary Janssen Pharmaceuticals. The drug is designed to be used in combination with diet and exercise to lower blood sugar levels in Type 2 diabetes patients. The drug is a sodium-glucose cotransporter-2 (SGLT2) inhibitor that causes sugar to be eliminated through the urine.
Invokana was first approved for sale by the FDA in March 2015. Within three months the FDA had 100 reports of Ketoacidosis and kidney damage in Invokana users. In May 2015, the FDA ordered the label contain a
“Some of these patients are otherwise healthy but for this terrible injury all of a sudden. They have been on this drug for a few months, or perhaps a few years and now they are seeing this Box Warning and putting two and two together,” McCormick says. “All of a sudden this is happening. No one told them about this and it would have changed how they handled their diabetes.”
The Invokana lawsuits have been consolidated in an MDL in New Jersey. The cases are going through case management process with discovery expected at a later date. It will be some time before the cases go to trial.
READ MORE
READ MORE Diabetes Drugs Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News

READER COMMENTS
Linda M Blackburn
on
Sarah Pickett
on
george ohsner
on
Donald Day
on
Jack Rosema
on
Melinda
on
Ohio
on
California
on
California
on
New York
on
.
Texas
on
Michigan
on
Ontario
on
Florida
on
Alabama
on
North Carolina
on
Tennessee
on