LAWSUITS NEWS & LEGAL INFORMATION
Victoza Side Effects
In March 2013 the US Food and Drug Administration (FDA) announced results of a study suggesting Victoza side effects could include an increased risk of Victoza pancreatitis. Victoza has already reportedly been linked in animal studies to an increased risk of a rare thyroid cancer, according to the FDA. So far, no Victoza lawsuits have been filed, although other drugs in the same medication class are the subject of lawsuits.
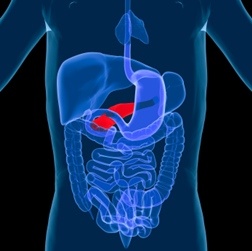 On March 14, 2013, the FDA issued a Drug Safety Communication, alerting the public to a study that suggested Victoza and other type 2 diabetes medications were linked to an increased risk of pancreatitis and pre-cancerous cellular changes, called pancreatic duct metaplasia. These risks were reportedly found in a group of drugs known as incretin mimetics, which includes Byetta, Bydureon, Victoza, Januvia and other medications.
On March 14, 2013, the FDA issued a Drug Safety Communication, alerting the public to a study that suggested Victoza and other type 2 diabetes medications were linked to an increased risk of pancreatitis and pre-cancerous cellular changes, called pancreatic duct metaplasia. These risks were reportedly found in a group of drugs known as incretin mimetics, which includes Byetta, Bydureon, Victoza, Januvia and other medications.
At the time, the FDA said it was not changing the safety warnings of the drugs, but that it was reviewing data from the study, including requesting the methodology used so the agency could investigate the risk of pancreatic toxicity.
Other drugs in the incretin mimetic class have been linked to an increased risk of acute pancreatitis, including fatal and nonfatal instances of the condition. When Victoza was approved, the FDA noted that in five clinical trials of Victoza, there were seven cases of pancreatitis in patients using Victoza, compared with one case of pancreatitis in a patient who used a different diabetes medicine.
On April 19, 2012, the public advocacy group Public Citizen filed a petition with the FDA requesting the agency remove Victoza from the market. Public Citizen cited the risk of thyroid cancer, pancreatitis, serious allergic reactions and kidney failure as outweighing any possible benefits to the drug. In a news release, Public Citizen noted that two FDA pharmacologists and an FDA clinical safety reviewer advised against approving Victoza.
Victoza, known generically as liraglutide, is used to treat type 2 diabetes and is made by Novo Nordisk. It was approved by the FDA in 2010 to treat bype 2 diabetes. When Victoza was approved, the FDA noted that animal data suggested a rare type of thyroid cancer, known as medullary thyroid cancer, was linked to the use of Victoza. According to the FDA, studies in mice and rats showed liraglutide was linked to the development of tumors in the thyroid gland, "especially at doses that were 8-times higher than what humans would receive."
As a result, the FDA requested a Risk Evaluation and Mitigation Strategy for Victoza and further required a five-year epidemiological study of Victoza.
Last updated on
FREE VICTOZA CANCER LAWSUIT EVALUATION
Send your Victoza Cancer claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Victoza Pancreatic Cancer
 On March 14, 2013, the FDA issued a Drug Safety Communication, alerting the public to a study that suggested Victoza and other type 2 diabetes medications were linked to an increased risk of pancreatitis and pre-cancerous cellular changes, called pancreatic duct metaplasia. These risks were reportedly found in a group of drugs known as incretin mimetics, which includes Byetta, Bydureon, Victoza, Januvia and other medications.
On March 14, 2013, the FDA issued a Drug Safety Communication, alerting the public to a study that suggested Victoza and other type 2 diabetes medications were linked to an increased risk of pancreatitis and pre-cancerous cellular changes, called pancreatic duct metaplasia. These risks were reportedly found in a group of drugs known as incretin mimetics, which includes Byetta, Bydureon, Victoza, Januvia and other medications.At the time, the FDA said it was not changing the safety warnings of the drugs, but that it was reviewing data from the study, including requesting the methodology used so the agency could investigate the risk of pancreatic toxicity.
Other drugs in the incretin mimetic class have been linked to an increased risk of acute pancreatitis, including fatal and nonfatal instances of the condition. When Victoza was approved, the FDA noted that in five clinical trials of Victoza, there were seven cases of pancreatitis in patients using Victoza, compared with one case of pancreatitis in a patient who used a different diabetes medicine.
Victoza Thyroid Cancer
Victoza, known generically as liraglutide, is used to treat type 2 diabetes and is made by Novo Nordisk. It was approved by the FDA in 2010 to treat bype 2 diabetes. When Victoza was approved, the FDA noted that animal data suggested a rare type of thyroid cancer, known as medullary thyroid cancer, was linked to the use of Victoza. According to the FDA, studies in mice and rats showed liraglutide was linked to the development of tumors in the thyroid gland, "especially at doses that were 8-times higher than what humans would receive."
As a result, the FDA requested a Risk Evaluation and Mitigation Strategy for Victoza and further required a five-year epidemiological study of Victoza.
Victoza Cancer Legal Help
If you or a loved one has suffered damages or injuries from Victoza, please click the link below and your complaint will be sent to a Drug/Medical Device lawyer who may evaluate your claim at no cost or obligation.Last updated on
VICTOZA CANCER LEGAL ARTICLES AND INTERVIEWS
Victoza: EMA Moving Forward While FDA Idles Over Victoza and Cancer

Opinions Split on Victoza
FDA Plays Down Victoza Cancer Concern, but Plaintiffs Beg to Differ


May 30, 2014
While cautious concern still exists with regard to Victoza side effects, the European Medicines Agency (EMA) has begun the process of actually allowing for expanded use of Victoza in the areas of the world governed by the EMA. READ MORE
Opinions Split on Victoza

May 1, 2014
Concerns about Victoza side effects, including a reported possible link between Victoza and cancer, have led to some critics requesting the type 2 diabetes drug be taken off the market. Not all studies, however, have confirmed the risk of cancer in patients taking Victoza. READ MORE
FDA Plays Down Victoza Cancer Concern, but Plaintiffs Beg to Differ

March 19, 2014
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) may have agreed that there isn’t a lot to talk about with regard to Victoza side effects. To wit, the two health regulators have parked any provocative concern over pancreatitis and precancerous changes to the pancreas with regard to Victoza and other drugs in the incretin mimetics class. But that hasn’t stopped lawsuits brought by plaintiffs alleging Victoza and other similarly classed drugs did in their pancreas. And now the FDA is investigating another drug in the class over a concern for heart failure. READ MORE
READ MORE Diabetes Drugs Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News

READER COMMENTS
Sherry Sewell
on
Luis mercado
on
I will stop the medicine and inform my doctor I do not want Victoza!!
I take metformin and lantus which I hope is safe!
Ruthie Thompson
on
Weight loss she said.I have nothing but constipation since I been on this meds.Took my self off 2/weeks now.Going to a new doctor now.Took blood work go back on the 10/28/2015.will have new doctor check my thyroids,my pancreatic,and what ever else to make sure no Damage has occurred,
latissia
on
Rosy
on
Now, I have been to the ER twice, missed 1 week of work and school. I stopped taking it 2 days ago on my own, but still having severe pain in right abdomen, back, bruising, and weakness all over my body. I will probably end up in ER tonight. This is ridiculous. I can't believe they would give this medication out with these types of side effects!!
Tony
on
Marta Orobitg
on
Georgia
on
Anonymous
on
Oklahoma
on
New Jersey
on
Illinois
on
New Jersey
on