LAWSUITS NEWS & LEGAL INFORMATION
Zocor Legal News Articles & Interviews
Zocor and Simvastatin Hit with New FDA Restrictions
 June 9, 2011. By Gordon Gibb.
June 9, 2011. By Gordon Gibb.FDA Issues Warning Regarding Zocor
March 19, 2010. By Heidi Turner.
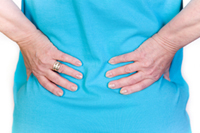
Silver Spring, MD The US Food and Drug Administration (FDA) issued a warning about the potential for an increased risk of muscle injury when patients use 80 mg of Zocor (simvastatin). The muscle injury, known as myopathy, is a side effect of all statins; however, the warning highlights the increased risk of developing this injury when patients use higher doses of Zocor. The warning includes an increased risk of rhabdomyolysis, a serious form of myopathy that can lead to kidney damage, kidney failure and death.
Read [ FDA Issues Warning Regarding Zocor ]
