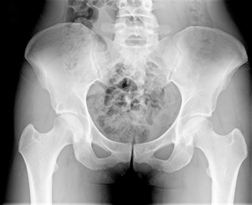
 The recall was caused by the high rate of hip revision surgeries needed by patients with DePuy hip replacements, according to Dow Jones Newswires.
The recall was caused by the high rate of hip revision surgeries needed by patients with DePuy hip replacements, according to Dow Jones Newswires.READ MORE DEPUY HIP REPLACEMENT LEGAL NEWS
According to the company, very few of these hip devices are still on the market, as they were discontinued last year, reports the news source.
Specifically, the parts that are being recalled are the ASR XL Acetabular System, the cup portion of a replacement joint and the ASR Hip Resurfacing System.
The recall was spurred by data that showed a hip revision surgery rate of about 13 percent for those with the ASR XL system and of about 12 percent for those with the ASR resurfacing system, reports the news provider.
