LAWSUITS NEWS & LEGAL INFORMATION
Bjork Shiley Heart Valve Legal News Articles & Interviews
Bjork-Shiley Heart Valve: Was Shiley Unethical in its Handling of the Fractures?
 December 18, 2012. By Heidi Turner.
December 18, 2012. By Heidi Turner.Bjork Shiley Lawsuits Settled Decades Ago, Could More be Filed?
 October 26, 2012. By Heidi Turner.
October 26, 2012. By Heidi Turner.Who Actually Pulled Bjork Shiley BSCC Heart Valve Off the Market?
 September 24, 2012. By Gordon Gibb.
September 24, 2012. By Gordon Gibb.Patients At Risk of Bjork Shiley Heart Valve Failure 25 Years After Removal from Market
.jpg) August 22, 2012. By Heidi Turner.
August 22, 2012. By Heidi Turner.Bjork Shiley Heart Valve Plagued by Deception: Report
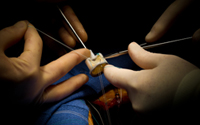 July 24, 2012. By Gordon Gibb.
July 24, 2012. By Gordon Gibb.Did Patients Receive Defective Bjork Shiley Heart Valves?
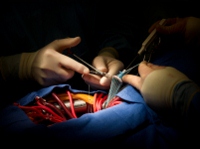 June 10, 2012. By Heidi Turner.
June 10, 2012. By Heidi Turner.Phantom Employee Used to Cover Bjork Shiley Heart Valve Defects
 May 9, 2012. By Heidi Turner.
May 9, 2012. By Heidi Turner.Philanthropic Generosity Cold Comfort to Bjork Shiley Heart Valve Patients
 April 18, 2012. By Gordon Gibb.
April 18, 2012. By Gordon Gibb.Patients Hurt by Deceit in Bjork Shiley Valve Approval
 March 10, 2012. By Heidi Turner.
March 10, 2012. By Heidi Turner.Patients Left with Bjork Shiley Valves Implanted
 February 10, 2012. By Heidi Turner.
February 10, 2012. By Heidi Turner.- Bjork Shiley Heart Valve a Ticking Time Bomb for Some By Gordon Gibb (Jan-25-12)
- Lethal Defects Of Bjork-Shiley Heart Valve By Evelyn Pringle (Apr-24-06)
