LAWSUITS NEWS & LEGAL INFORMATION
Topamax Birth Defects
Read our Topamax FAQ
The US Food and Drug Administration (FDA) has issued a warning about the risk of Topamax side effects including a risk of Topamax birth defects in children exposed to Topamax prior to birth. These Topamax oral birth defects include a risk of cleft lip and cleft palate. Because of the risk of Topamax cleft palate or cleft lip, the FDA has requested that Topamax carry a stronger warning and announced it will change Topamax from Pregnancy Category C to Pregnancy Category D.
 Topamax, known generically as topiramate, is approved by the FDA to treat certain types of seizures and to prevent migraine headaches. Based on new research, the FDA suggests that health care professionals strongly weigh the benefits and risks of topiramate when prescribing it to women who are of childbearing age, and Topamax will now be classified as a Pregnancy Category D drug. Part of the problem is that oral clefts occur when parts of the lip or palate do not completely fuse in the first trimester of pregnancy—before some women even know they are pregnant.
Topamax, known generically as topiramate, is approved by the FDA to treat certain types of seizures and to prevent migraine headaches. Based on new research, the FDA suggests that health care professionals strongly weigh the benefits and risks of topiramate when prescribing it to women who are of childbearing age, and Topamax will now be classified as a Pregnancy Category D drug. Part of the problem is that oral clefts occur when parts of the lip or palate do not completely fuse in the first trimester of pregnancy—before some women even know they are pregnant.
 In making its announcement, the FDA cited research from the North American Antiepileptic Drug (AED) Pregnancy Registry, which suggested an increased risk of oral clefts—including cleft lip and cleft palate—in infants who were exposed to Topamax and its generic counterparts during the first trimester. According to the data, infants who were exposed to topiramate experienced a 1.4 percent prevalence of oral clefts, whereas infants who were exposed to other antiepileptic medications had 0.38 - 0.55 percent prevalence. Infants who are not exposed to any antiepileptic medications had a 0.07 percent prevalence of oral cleft.
In making its announcement, the FDA cited research from the North American Antiepileptic Drug (AED) Pregnancy Registry, which suggested an increased risk of oral clefts—including cleft lip and cleft palate—in infants who were exposed to Topamax and its generic counterparts during the first trimester. According to the data, infants who were exposed to topiramate experienced a 1.4 percent prevalence of oral clefts, whereas infants who were exposed to other antiepileptic medications had 0.38 - 0.55 percent prevalence. Infants who are not exposed to any antiepileptic medications had a 0.07 percent prevalence of oral cleft.
Following an analysis of this data, the FDA has determined that Topamax will be moved from Pregnancy Category C to Pregnancy Category D. Pregnancy Category D means that there is evidence of human fetal risk based on human data but the benefits of the drug in some pregnant women may still outweigh the risks.
In addition to the risk of Topamax cleft palate or cleft lip, there are a number of other potential Topamax side effects. Some of these include:
 The patient medication guide and the prescribing information for Topamax will be updated to provide patients with the new information regarding Topamax birth defects--particularly Topamax cleft lip and topamax cleft palate.
The patient medication guide and the prescribing information for Topamax will be updated to provide patients with the new information regarding Topamax birth defects--particularly Topamax cleft lip and topamax cleft palate.
Oral cleft defects, which include cleft lip and cleft palate, can lead to problems with eating, talking, and to ear infections. Surgery often is performed to close the lip and palate and most children do well after treatment.
Women who are pregnant or planning on becoming pregnant and taking Topamax or topiramate should speak with their doctors to discuss their treatment options.
Topamax is sold by Johnson & Johnson but is also available in generic versions. Johnson & Johnson said it is working with the FDA to update the label.
In April 2011, Johnson & Johnson recalled two lots of Topamax after receiving complaints about an uncharacteristic odor from the medication. According to The Wall Street Journal (04/14/11), the Topamax recall affects approximately 57,000 bottles of 100 milligram Topamax tablets, shipped in October and December 2010. The odor that resulted in the Topamax recall was linked to temporary gastrointestinal symptoms but no reports of serious health effects.
The problem is reportedly linked to chemically-treated wooden pallets. The company is investigating whether other products were affected by the same problem, believed caused by TBA, a chemical used to treat wooden pallets.
Last updated on
FREE TOPAMAX LAWSUIT EVALUATION
Send your Topamax claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Topamax And Pregnancy
 Topamax, known generically as topiramate, is approved by the FDA to treat certain types of seizures and to prevent migraine headaches. Based on new research, the FDA suggests that health care professionals strongly weigh the benefits and risks of topiramate when prescribing it to women who are of childbearing age, and Topamax will now be classified as a Pregnancy Category D drug. Part of the problem is that oral clefts occur when parts of the lip or palate do not completely fuse in the first trimester of pregnancy—before some women even know they are pregnant.
Topamax, known generically as topiramate, is approved by the FDA to treat certain types of seizures and to prevent migraine headaches. Based on new research, the FDA suggests that health care professionals strongly weigh the benefits and risks of topiramate when prescribing it to women who are of childbearing age, and Topamax will now be classified as a Pregnancy Category D drug. Part of the problem is that oral clefts occur when parts of the lip or palate do not completely fuse in the first trimester of pregnancy—before some women even know they are pregnant.
Topamax Cleft Palate and Cleft Lip
 In making its announcement, the FDA cited research from the North American Antiepileptic Drug (AED) Pregnancy Registry, which suggested an increased risk of oral clefts—including cleft lip and cleft palate—in infants who were exposed to Topamax and its generic counterparts during the first trimester. According to the data, infants who were exposed to topiramate experienced a 1.4 percent prevalence of oral clefts, whereas infants who were exposed to other antiepileptic medications had 0.38 - 0.55 percent prevalence. Infants who are not exposed to any antiepileptic medications had a 0.07 percent prevalence of oral cleft.
In making its announcement, the FDA cited research from the North American Antiepileptic Drug (AED) Pregnancy Registry, which suggested an increased risk of oral clefts—including cleft lip and cleft palate—in infants who were exposed to Topamax and its generic counterparts during the first trimester. According to the data, infants who were exposed to topiramate experienced a 1.4 percent prevalence of oral clefts, whereas infants who were exposed to other antiepileptic medications had 0.38 - 0.55 percent prevalence. Infants who are not exposed to any antiepileptic medications had a 0.07 percent prevalence of oral cleft.
Following an analysis of this data, the FDA has determined that Topamax will be moved from Pregnancy Category C to Pregnancy Category D. Pregnancy Category D means that there is evidence of human fetal risk based on human data but the benefits of the drug in some pregnant women may still outweigh the risks.
Topamax Side Effects
- Constipation or diarrhea
- Dizziness
- Headache
- Nausea, stomach pain or upset stomach
- Flu-like symptoms, runny nose, sore throat
- Loss of appetite
- Severe allergic reactions such as rash; hives; itching; difficulty breathing; tightness in the chest, swelling of the mouth, face, lips, or tongue
- Blood in the urine
- Blurred vision, double vision or other vision changes, eye pain
- Bone pain, chest pain, muscle or joint pain, severe stomach, side or back pain
- Confusion, decreased coordination
- New or worsening mood changes: anxiety, depression, hostility
- Menstrual changes, cramps
- Red, swollen, peeling or blistered skin
- Irregular heartbeat, loss of consciousness
- Rapid, shallow breathing
- Severe or persistent loss of appetite, significant weight loss
- Suicidal thoughts or actions
- Tremors
- Trouble walking
- Unexplained fever
- Unusual bruising or bleeding
- Unusual eye movements
- Unusual tiredness
Topamax (Topiramate) Label Change for Cleft Lip & Cleft Palate
 The patient medication guide and the prescribing information for Topamax will be updated to provide patients with the new information regarding Topamax birth defects--particularly Topamax cleft lip and topamax cleft palate.
The patient medication guide and the prescribing information for Topamax will be updated to provide patients with the new information regarding Topamax birth defects--particularly Topamax cleft lip and topamax cleft palate.
Oral cleft defects, which include cleft lip and cleft palate, can lead to problems with eating, talking, and to ear infections. Surgery often is performed to close the lip and palate and most children do well after treatment.
Women who are pregnant or planning on becoming pregnant and taking Topamax or topiramate should speak with their doctors to discuss their treatment options.
Topamax is sold by Johnson & Johnson but is also available in generic versions. Johnson & Johnson said it is working with the FDA to update the label.
Topamax Recall
The problem is reportedly linked to chemically-treated wooden pallets. The company is investigating whether other products were affected by the same problem, believed caused by TBA, a chemical used to treat wooden pallets.
Topamax Cleft Palate & Topamax Cleft Lip Legal Help
If you or a loved one has suffered damages in this case as a result of taking Topamax or topiramate, please click the link below and your complaint will be sent to a lawyer who may evaluate your claim at no cost or obligation.Last updated on
TOPAMAX LEGAL ARTICLES AND INTERVIEWS
$10.9 Million Verdict Revived Against Topamax Maker Janssen Pharmaceutical

$11 Million Topamax Birth Defect Award Stands
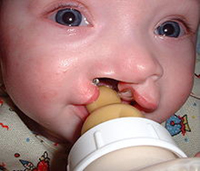
Another Victory for Plaintiffs in the Case against Topamax


June 18, 2016
A Pennsylvania appeals court affirmed the $10.9 million verdict against Johnson & Johnson’s Janssen Pharmaceuticals involving the anti-seizure drug Topamax that caused birth defects. READ MORE
$11 Million Topamax Birth Defect Award Stands

May 7, 2014
An $11 million jury award that went to a Topamax plaintiff in November of last year will stand following the decision of a Philadelphia lawmaker. Court of Common Pleas Judge George W. Overton, in denying Johnson & Johnson (J&J) subsidiary Janssen Pharmaceuticals’ motion for a new trial, upheld the Topamax birth defect jury award for the parents of Brayden Gurley. READ MORE
Another Victory for Plaintiffs in the Case against Topamax

March 12, 2014
A jury trial in Philadelphia has delivered yet another blow to the manufacturers of Topamax, and ordered Janssen Pharmaceuticals to pay $3 million in damages to Kelly and Brian Anderson and their child, who was born five years ago with a bilateral cleft palate and lip. READ MORE
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Anonymous
on
I was admitted so they could stress-test my heart and do an echo. Normal. Except for frequent PVC PAC and short bursts of SVT. I was given lopressor with some reduction in chest discomfort, but my weakness and confusion and inability to function just got worse. I took a medical leave from work and saw an arrhythmia specialist . Lopressor was increased to 50 mg. I got to the point I was sleeping 20 hours of the day.
As a health care provider I try not to manage my own care as it can complicate clear diagnoses etc..-- so as a rule I stay off the Internet. But when I realized I wasn't getting any better I accessed all my labs that were available through a patient access site. I noticed that for the last 6 months my serum carbon dioxide was low and my chloride was high. I brought it to one doctors attention and was told by her nurse that maybe I hyper ventilate when they draw my blood. Then , probably because I was so confused, I actually put aside the labs and stopped my investigation after that smack down.
Another couple weeks of sleep went by and in a moment of frustration I finally just googled topamax low serum carbon dioxide , high chloride. I was still kind of not thinking straight and did not know what to do so I just stayed home hoping I would wake up when I went to sleep. I was so mixed up and felt so unsure that I was afraid to sound like a crazy person-- because I had sounded like a crazy person increasingly over the last couple years. I was afraid to stop the topamax because I was now taking lopressor to suppress the arrhythmia and how do you explain all that over the phone to a doctor who doesn't know you. So I went to the ED. The doctor saw I had been an inpatient twice and was being treated with lopressor and asked me "why would you come here? What do you think I can do for you?" I explained my fears and asked his advice and he did an ABG and a metabolic panel. They showed metabolic acidosis. He told me coming to the ED was the wrong venue and sent me on my way in tears. I am presently weaning off topamax with my primary care supervising. Follow-up labs will be done and I hope no permanent damage was done. I was considering having an ablation for the arrhythmia.