 After barely 18 months on the market, the FDA was compelled to mandate a black box warning for Incivek this past December, after reports of serious skin rash that have since been associated with SJS. Specifically, the FDA noted that a patient in Japan had died from toxic epidermal necrolysis (TEN), which is the most serious form of Stevens Johnson Syndrome rash. The patient had continued with Incivek combination treatment after developing the rash.
After barely 18 months on the market, the FDA was compelled to mandate a black box warning for Incivek this past December, after reports of serious skin rash that have since been associated with SJS. Specifically, the FDA noted that a patient in Japan had died from toxic epidermal necrolysis (TEN), which is the most serious form of Stevens Johnson Syndrome rash. The patient had continued with Incivek combination treatment after developing the rash.The black box warning requires any patient on Incivek combination treatment to heed the appearance of an emerging skin rash as a serious warning sign and stop Incivek immediately.
According to the official FDA website, Incivek is a hepatic C virus NS3/4A protease inhibitor indicated in combination with peginterferon alfa and ribavirin for the treatment of genotype 1 chronic hepatitis C in adult patients with compensated liver disease, including patients who have cirrhosis, are treatment-naïve, or who have previously received interferon-based treatment. The treat protocol holds that Incivek must always be used in combination with peginterferon alfa and ribavirin.
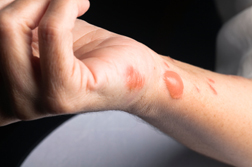
Stevens Johnson Syndrome skin disease is an allergic reaction to medication that directly affects the skin. Beginning as a rash, SJS can quickly escalate to blisters and lesions that can be likened to serious burns. SJS rash patients, in fact, are usually treated in burn units, as the characteristic of the skin associated with SJS and TEN remains very similar to burn injuries.
While the association with Stevens Johnson syndrome symptoms appears to be a recent development, skin rash is by no means new. In fact, according to the FDA’s own safety announcement released December 19 last year, “Incivek’s manufacturer, Vertex Pharmaceuticals Incorporated, agreed at the time of marketing approval to investigate, through genetic analysis, the factors associated with serious skin reactions following Incivek combination treatment.”
The suggestion is that potential for serious skin rash was enough of a concern at the point when Incivek was initially approved, that Vertex was asked to continue investigating. The assumption that the FDA approved Incivek while knowing the risk for serious skin rash is in keeping with the FDA’s mandate for approval of drugs where the benefits significantly outweigh the risks for the intended patient. The promise for hepatitis C patients of Incivek was articulated by The New York Times in 2011, when the venerable paper reported that in a clinical trial, 79 percent of trial participants treated with Incivek in combination with existing treatment “achieved what is effectively considered a cure,” compared with 46 percent without Incivek.
Promising indeed. However, the FDA’s recent black box warning update for Incivek related to skin rash and Stevens Johnson Syndrome symptoms appears to curtail the previously heady benefits profile for Incivek, in directing physicians to immediately stop Incivek combination treatment at the first sign of skin trouble. So much for the benefit outweighing the risk, and the awareness of potential skin rash at the time Incivek was approved.
It should be noted that two months after the FDA issued its safety alert, Health Canada has issued a similar warning, according to RTTNews (3/1/13). The directive, issued February 27 in Canada, does not appear to advocate stopping Incivek treatment entirely, but instead advocates for an immediate consultation with a doctor at the first sign of a skin rash, as well as itching, mouth sores or ulcers, red or inflamed eyes, or fever.
The FDA, for its part, was more blunt. “If serious skin reactions occur, all three components of Incivek combination treatment, including peginterferon alfa and ribavirin, must be immediately discontinued, and the patient should receive urgent medical care.”
READ MORE STEVENS JOHNSON SYNDROME (SJS) LEGAL NEWS
Hepatitis C patients who have experienced problems with SJS Incivek way wish to pursue a case with Stevens Johnson Syndrome lawyers.

READER COMMENTS
Stout Marian
on
Anthony Sugel
on
John MacNeil
on
Domenica Rivera
on