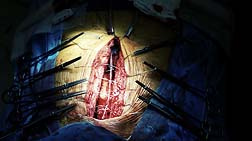
 The problem lay with the tricky surgery necessary to extract a lead, a procedure that is fraught with risk. A heart lead will often be overgrown, or entwined with tissue. Thus, removing a heart lead—regardless of whether it is a working lead, or one that has proved to fail—is an exact and dangerous procedure that can't be done by just anybody.
The problem lay with the tricky surgery necessary to extract a lead, a procedure that is fraught with risk. A heart lead will often be overgrown, or entwined with tissue. Thus, removing a heart lead—regardless of whether it is a working lead, or one that has proved to fail—is an exact and dangerous procedure that can't be done by just anybody.In other words, the successful removal of a heart lead will be accomplished by a surgeon with a great deal of experience doing just that. Sometimes, surgeons with those qualifications are hard to come by.
Medtronic had a hit on its hands when the Sprint Fidelis lead was first introduced onto the market in 2004. Thinner than its competitors, surgeons found it easier to thread to the heart and it wasn't long before a quarter of a million people around the world were walking around with Sprint Fidelis leads. In the US, that figure is around 150,000.
But then came the failures. The Sprint Fidelis lead was found to be prone to cracking, which affects the communication pathway to the heart. In the case of a defibrillator, this breach has resulted in a failing heart not getting the proper electronic prompting it needs to get it going again. Worse, there have been cases where such a breach in the wire has resulted in the defibrillator getting an incorrect signal, and thinking that the heart was failing, delivered a life-sustaining electrical pulse to what was in reality a properly-functioning heart.
Some patients have died. Others have had their health compromised after a pacemaker, connected to a Sprint Fidelis lead, was incapable of helping the heart maintain a correct rhythm.
Medtronic has said that the failure rate is about 5 percent, 45 months into the life of a lead. And even though many patients are finding that their leads are, indeed functioning properly, many patients who cannot live with the prospect of the lead potentially failing are opting to have them replaced proactively.
"I think we are just seeing the tip of the iceberg," said Dr. Charles J. Love, a cardiologist at Ohio State University Medical Center in Columbus, who specializes in cable extractions, in comments published in the New York Times.
However, as more and more patients opt for the risky procedure, the death toll may rise given the inherent risk to the heart and / or arteries when an extraction is attempted. Already, four patients have died during extraction procedures, and industry watchers fear that those numbers will rise as more patients seek extractions from a medical community that has limited expertise in the risky extraction process.
The risk is such that many surgeons, when replacing a heart lead, will often leave the old lead in place—threading the new lead alongside.
There are diverging opinions and positions as to what to do when a pacemaker, or defibrillator itself needs to be updated once the batteries wear out, usually after five years. Some surgeons will reconnect to the existing Sprint Fidelis lead if it is still functioning.
Others, including Dr. Love in Ohio State, are routinely replacing the Sprint Fidelis leads when pacemakers or defibrillators are in need of updating in younger, more active patients. Typically, those patients are age 60 years of age or lower. The reason? Greater physical activity places more stress on a cable, which raises the likelihood of fracture.
Critics of the Sprint Fidelis lead cite that very fact as to why, in part, the lead should never have been approved in the first place. As the baby boomers age, the country is seeing a more active senior. Using a thinner lead in concert with an active individual—heart problems aside—just doesn't seem to make a lot of sense.
READ MORE Sprint Fidelis LEGAL NEWS
However, critics cite that the Sprint Fidelis lead was approved without adequate FDA testing.
While Medtronic will supply replacement heart leads, the cost of the operation to implant, and / or extract an existing lead can cost $15,000 to $20,000. That cost must be borne by Medicare, a private HMO, or the patient.
The next few years will prove interesting.
