LAWSUITS NEWS & LEGAL INFORMATION
Medtronic CD Horizon Legal News Articles & Interviews
Medtronic CD Horizon: Caveat Emptor
 October 14, 2009. By Jane Mundy.
October 14, 2009. By Jane Mundy.Patient must have Medtronic CD Horizon spinal system Removed
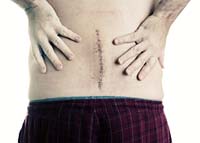 October 1, 2009. By Jane Mundy.
October 1, 2009. By Jane Mundy.Failed CD Horizon System Fast-Tracked to Approval, Manufacturer Played by the Rules
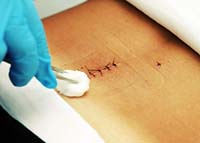 September 22, 2009. By Gordon Gibb.
September 22, 2009. By Gordon Gibb.