LAWSUITS NEWS & LEGAL INFORMATION
Invokana Legal News Articles & Interviews
Plaintiff Blames Loss of his Lower Leg to Invokana Use
 March 21, 2018. By Gordon Gibb.
March 21, 2018. By Gordon Gibb.Trenton, NJ: While a plaintiff having filed a recent Invokana lawsuit makes assertions pertaining to Invokana linked with cardiovascular injuries and kidney failure, it was the amputation of his right leg below the knee less than a year after starting on Invokana that ultimately prompted the plaintiff to move forward with his Invokana side effects lawsuit.
Read [ Plaintiff Blames Loss of his Lower Leg to Invokana Use ]
New Invokana Research (Funded by Manufacturer) Disputes Earlier Studies Showing Amputation Risk
 October 2, 2017. By Jane Mundy.
October 2, 2017. By Jane Mundy.Washington, DC: Despite exhaustive studies that found an increased risk of amputation among Invokana diabetes patients, Janssen Pharmaceuticals has published its own version of research.
Read [ New Invokana Research (Funded by Manufacturer) Disputes Earlier Studies Showing Amputation Risk ]
Janumet Patient Felled by Cancer of the Pancreas in 2012
 August 22, 2017. By Gordon Gibb.
August 22, 2017. By Gordon Gibb.Los Angeles, CA: When Janumet (sitagliptin and metformin combined) was approved by the US Food and Drug Administration (FDA) in 2007, the type 2 diabetes drug was within five years of achieving sales of $1.7 Billion. That year, when Janumet hit blockbuster status by 2012, the parent drug of Janumet – Januvia – earned $4 Billion for manufacturer Merck & Co. Januvia has been associated with Januvia side effects, and has resulted in Januvia cancer lawsuits.
Read [ Janumet Patient Felled by Cancer of the Pancreas in 2012 ]
Are Risk of Invokana and Invokamet Bone Fractures a Sign of Fractured Regulations?
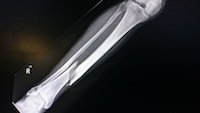 August 8, 2017. By Gordon Gibb.
August 8, 2017. By Gordon Gibb.Washington, DC: A drug indicated for the treatment of type 2 diabetes already carries warnings for compelling adverse reactions including a heightened risk for lower limb amputations, kidney failure, diabetic ketoacidosis and cardiovascular injuries. To that end, the FDA warns of fracture risk with Invokana and Invokamet. Yet in spite of such serious side effects associated with Invokana (canagliflozin) and Invokamet (canagliflozin and metformin), the US Food and Drug Administration (FDA) approved, last year an expanded indication for Invokamet.
Read [ Are Risk of Invokana and Invokamet Bone Fractures a Sign of Fractured Regulations? ]
FDA Black Box Warning Adds Fuel to Invokana Lawsuits
 June 24, 2017. By Brenda Craig.
June 24, 2017. By Brenda Craig.Philadelphia, PA Final results from two independent clinical trials indicate the amputation risk for users of the Type 2 Diabetes drug Canagliflozin, marketed as Invokana, Invokamet, Invokamet XR, are much greater than previously understood.
The FDA was so concerned about the information the studies provided that it moved immediately to ensure consumers are warned about the increased risk of leg and foot amputations through a Boxed Warning label on all Invokana prescriptions.
“I think the fact that the independent clinical trials lead to a situation where the FDA went straight to a black box warning, its highest level of scrutiny, just shows how important and how critical this information is to the public,” says Brian J. McCormick Jr., a Philadelphia attorney from the firm of Ross Feller Casey.
To McCormick, Jr. the studies confirm and add significant weight to the many personal injury claims he and his firm are bringing forward on behalf of diabetic Americans that allege they have been injured as a result of using Invokana to control their Type 2 Diabetes Mellitus.
The studies (CANVAS Canagliflozin Cardiovascular Assessment Study) and the CANVAS-R (A Study of the Effects of Canagliflozin on Renal Endpoints in Adult Participants with Type 2 Diabetes Mellitus) showed, according to a FDA website safety warning (May 2017), that leg and foot amputation occurred about twice as often in patients treated with Canagliflozin compared to patients taking a placebo.
“It is a terrible progression for some of these patients,” says McCormick. “They go in and they have a diabetic foot ulcer and they don’t how bad it is. They have one amputation and then they go back and have another toe removed – and next thing they know they have half their foot removed.”
According to the FDA safety communication on May 15, 2017, “Amputations of the toe and middle of the foot were the most common; however, amputations involving the leg, below and above the knee, also occurred. Some patients had more than one amputation, some involving both limbs.”
“Our firm is investigating both Ketoacidosis cases and we are also investigating amputation cases,” says McCormick. “We have a number of clients we are working with and I think at the end of the day what this new information shows, and will show in court, is that Johnson & Johnson did know that this risk existed and chose not to do anything about it.”
Canagliflozin is licensed for sale in North America by Johnson & Johnson subsidiary Janssen Pharmaceuticals. The drug is designed to be used in combination with diet and exercise to lower blood sugar levels in Type 2 diabetes patients. The drug is a sodium-glucose cotransporter-2 (SGLT2) inhibitor that causes sugar to be eliminated through the urine.
Invokana was first approved for sale by the FDA in March 2015. Within three months the FDA had 100 reports of Ketoacidosis and kidney damage in Invokana users. In May 2015, the FDA ordered the label contain a
“Some of these patients are otherwise healthy but for this terrible injury all of a sudden. They have been on this drug for a few months, or perhaps a few years and now they are seeing this Box Warning and putting two and two together,” McCormick says. “All of a sudden this is happening. No one told them about this and it would have changed how they handled their diabetes.”
The Invokana lawsuits have been consolidated in an MDL in New Jersey. The cases are going through case management process with discovery expected at a later date. It will be some time before the cases go to trial.
Invokana Adverse Events Subject to Much Scrutiny
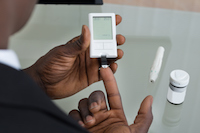 June 13, 2017. By Gordon Gibb.
June 13, 2017. By Gordon Gibb.Boston, MA: A new study recently published in the New England Journal of Medicine (06/08/17) with regard to the potential for diabetic ketoacidosis (DKA) shows that type 2 diabetes patients on sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs appear to have double the risk for DKA when compared with patients using dipeptidyl peptidase-4 (DPP-4) inhibitors. Invokana is a member of the SGLT2 class, and ample concern has been expressed over Invokana linked with cardiovascular injuries and kidney failure.
Read [ Invokana Adverse Events Subject to Much Scrutiny ]
Invokana to Get Black-Box Warning over Amputation Risk
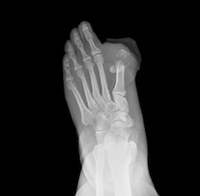 May 18, 2017. By Gordon Gibb.
May 18, 2017. By Gordon Gibb.Washington, DC: An issue that looms even larger than that of Invokana linked with cardiovascular injuries and kidney failure is the potential for amputation associated with the diabetes drug. The issue first surfaced a year ago when the US Food and Drug Administration (FDA) issued an alert after studying the results of two clinical trials, warning that users of Invokana and Invokamet were twice as likely to require amputations of appendages, or limbs.
Read [ Invokana to Get Black-Box Warning over Amputation Risk ]
Invokana Lawsuits Keep Trickling In
 April 15, 2017. By Gordon Gibb.
April 15, 2017. By Gordon Gibb.Trenton, NJ: We’re coming up to the first anniversary of a health and safety review conducted by Health Canada in May, 2016 with regard to Sodium-glucose cotransporter-2 (SGLT2) inhibitors: Invokana (canagliflozin), Forxiga (dapagliflozin), and Jardiance (empagliflozin). The move followed a similar review and a warning by the US Food and Drug Administration (FDA) with regard to the potential for cardiovascular injuries and kidney failure.
Read [ Invokana Lawsuits Keep Trickling In ]
Invokana Lawsuits Will Remain in Federal Court In Philadelphia
 March 8, 2017. By Gordon Gibb.
March 8, 2017. By Gordon Gibb.Philadelphia, PA: A series of complex legal arguments and maneuvering with regard to jurisdictional issues may have served to briefly divert attention away from Invokana adverse events. However, plaintiffs are nonetheless steeled in their resolve to pursue compensation amidst allegations over Invokana linked with cardiovascular injuries and kidney failure.
Read [ Invokana Lawsuits Will Remain in Federal Court In Philadelphia ]
Invokana Still Invoking Concerns
 February 8, 2017. By Gordon Gibb.
February 8, 2017. By Gordon Gibb.Trenton, NJ: It was about a year ago that the European Medicines Agency (EMA) raised the alarm over kidney problems associated with diabetes medication Invokana and other drugs in the SGLT2 class. This followed a warning the previous year from the Institute for Safe Medication Practices, which reported in 2015 its identification of some 457 serious adverse events involving kidney failure or impairment. Dehydration and fluid imbalances, kidney stones, urinary tract infections and abnormal weight loss were also identified. That led to various headlines suggesting Invokana Linked with Cardiovascular Injuries and Kidney Failure.
Read [ Invokana Still Invoking Concerns ]
- Plaintiffs Celebrate Invokana Lawsuit Consolidation By Heidi Turner (Jan-3-17)
- Judicial Panel Considers Consolidating Invokana Lawsuits By Heidi Turner (Dec-10-16)
- Janssen Loses One Invokana Battle By Heidi Turner (Nov-11-16)
- Plaintiffs Move to Consolidate Invokana Lawsuits By Heidi Turner (Oct-5-16)
- Invokana Lawsuits: Health Authorities Strengthen Warnings By Heidi Turner (Sep-6-16)
- Health Canada Updates Invokana Warning Ahead of the FDA By Gordon Gibb (Aug-1-16)
- FDA Strengthens Invokana Warnings By Heidi Turner (Jul-7-16)
- Invokana Floats Like a Butterfly, Stings Like a Bee By Gordon Gibb (Jun-5-16)
- Some Plaintiffs Develop DKA Mere Months after Starting Invokana By Gordon Gibb (May-31-16)
- More Invokana Lawsuits Filed By Heidi Turner (May-6-16)
- Diabetes Specialist Defends Invokana, Plaintiff Continues with Her Lawsuit Anyway By Gordon Gibb (Apr-3-16)
- Canadian Invokana Diabetes Patient Sues Janssen Pharmaceuticals By Brenda Craig (Mar-26-16)
- Invokana Goes to Court in Canada By Brenda Craig (Mar-24-16)
- Invokana Lawsuits Heating Up, Plaintiffs Allege Negligence By Gordon Gibb (Mar-8-16)
- Plaintiff Launches $1Billion Class-Action Invokana Lawsuit, Others Follow By Gordon Gibb (Feb-23-16)
- ISMP Questions Invokana Safety By Heidi Turner (Feb-12-16)
- Invokana Lawsuit Filed within days of Latest FDA Warning By Gordon Gibb (Jan-3-16)
- Invokana’s Deadly Side Effects Create the Opportunity for Major Recovery By The National Trial Lawyers (Dec-19-15)
- $1 Billion Invokana Lawsuit Filed in Canada By Heidi Turner (Dec-3-15)
- Consumer Group Concerned Over Off-Label Marketing of Invokana By Gordon Gibb (Nov-9-15)
- Additional Concerns Surface Over Invokana Side Effects: FDA By Gordon Gibb (Oct-4-15)
- FDA Warning Prompts Invokana Lawsuit Investigation By Heidi Turner (Aug-11-15)
- Invokana Problems Mount Just Two Years following FDA Approval By Gordon Gibb (Jul-6-15)
- Safety Review of SGLT2 Inhibitors Initiated by Health Canada By Lucy Campbell (Jun-22-15)
