LAWSUITS NEWS & LEGAL INFORMATION
Defective Products Legal News Articles & Interviews
Takata Airbag Recalls Increases by 35 Million Vehicles
 May 5, 2016. By Lucy Campbell.
May 5, 2016. By Lucy Campbell.Washington, DC: Federal regulators have announced that an additional 35 million Takata airbags will be recalled across the US, bringing the total recall up to 63 million, more than doubling the largest automotive recall in US history. Similarly, in Canada, several million vehicles will also be recalled.
Read [ Takata Airbag Recalls Increases by 35 Million Vehicles ]
Cancer Risk Prompts J&J Suspension of Fibroid Surgical Device
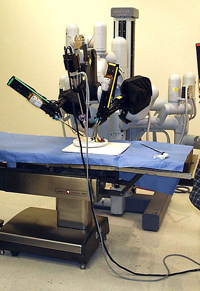 April 30, 2014. By Lucy Campbell.
April 30, 2014. By Lucy Campbell.Another Defect in da Vinci Robot
 May 11, 2013. By Jane Mundy.
May 11, 2013. By Jane Mundy.FDA Takes Control of Tylenol Plants
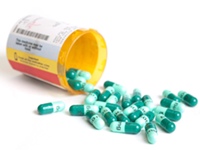 March 14, 2011. By Lucy Campbell.
March 14, 2011. By Lucy Campbell.All Marketed Sibutramine Drugs Voluntarily Withdrawn in Canada
 October 13, 2010. By Lucy Campbell.
October 13, 2010. By Lucy Campbell.ATV Riders 50 Percent More Likely to Die Following an ATV Accident
 October 6, 2010. By Lucy Campbell.
October 6, 2010. By Lucy Campbell.Court of Appeal Limits Impact of Federal Statute of Repose
 June 8, 2010. By Heidi Turner.
June 8, 2010. By Heidi Turner.New Pampers Product Alleged to Cause Severe Skin Rash
 May 12, 2010. By Gordon Gibb.
May 12, 2010. By Gordon Gibb.