LAWSUITS NEWS & LEGAL INFORMATION
Bisphosphonates Legal News Articles & Interviews
Did Merck Know About Fosamax Fracture Risk?
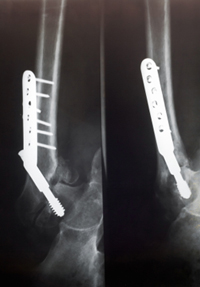 April 10, 2013. By Lucy Campbell.
April 10, 2013. By Lucy Campbell.Consumer Reports Concerned About Bisphosphonate Drugs
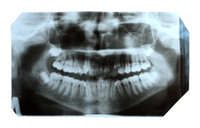 December 15, 2012. By Gordon Gibb.
December 15, 2012. By Gordon Gibb.Bisphosphonate Lawsuit Results in $10 Million Award
.jpg) November 23, 2012. By Heidi Turner.
November 23, 2012. By Heidi Turner.A Fosamax Femur Fracture After Just Three Years
.jpg) November 16, 2012. By Gordon Gibb.
November 16, 2012. By Gordon Gibb.The Catch 22 Associated With Bisphosphonate Drugs
.jpg) October 31, 2012. By Gordon Gibb.
October 31, 2012. By Gordon Gibb.LawyersandSettlements.com Alerts Public of Dangers of Popular Bone Loss Drugs including Fosamax
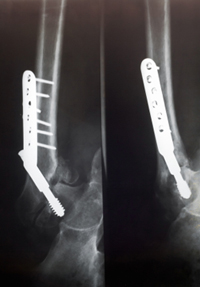 May 2, 2012. By LAS Staff Writer.
May 2, 2012. By LAS Staff Writer.With a steadily aging population, there has never been a greater focus on bone health and osteoporosis prevention. According to the National Osteoporosis Foundation(OPF), 10 million Americans currently suffer from brittle bones, with another 34 million exhibiting signs of low bone density. Women represent 80 percent of those affected, and nearly a quarter of individuals who break a hip do not survive beyond a year following their hip fracture. Given such statistics, osteoporosis prevention drugs, like Fosamax, have become increasingly popular, at the risk of potentially greater prevalence of Fosamax side effects.
More Fosamax Lawsuits Filed, despite Defendant Victories
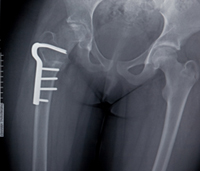 October 26, 2011. By Heidi Turner.
October 26, 2011. By Heidi Turner.FDA Panels Advise Label Clarification to Prevent Fosamax Side Effects
 September 19, 2011. By Charles Benson.
September 19, 2011. By Charles Benson.The Case for a Fosamax Holiday
 September 14, 2011. By Gordon Gibb.
September 14, 2011. By Gordon Gibb.