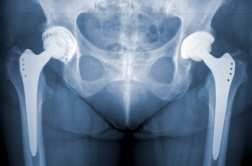
Maurice Brigham, who received two hip implants in 2007, filed a lawsuit in California alleging that officials in Johnson & Johnson's DePuy Orthopedics unit knew that many of the patients who received ASR hip implants suffered from defects in the devices and required second surgeries—known as hip revision surgery—to correct the problems.
According to Bloomberg on 9/03/10, the lawsuit was filed shortly after Johnson & Johnson announced a recall of the ASR XL Acetabular and the ASR Hip Resurfacing systems. The recall, announced August 26, 2010, was initiated after researchers found that after five years, 13 percent of patients who underwent complete hip replacements needed hip revision surgery. Furthermore, 12 percent of patients who had resurfacing systems also required hip revision surgeries.
Those unpublished statistics, cited by DePuy Orthopedics in a statement announcing the recall, came from data from the National Joint Registry (NJR) of England and Wales. The company noted that the revision rates are similar across the entire device size range.
READ MORE DEPUY HIP REPLACEMENT LEGAL NEWS
David Floyd, president of DePuy Orthopedics said the company is committed to paying for the cost of doctor visits, tests and procedures associated with the recall.
Approximately 93,000 units were affected by the recall. In 2009, DePuy discontinued the ASR system, citing declining demand.
Meanwhile, the Wall Street Journal reported on 8/26/10 that DePuy received a warning letter after marketing some replacement-joint products without the approval of the Federal Drug Administration (FDA). DePuy has said it would respond to the FDA's request for information.
