LAWSUITS NEWS & LEGAL INFORMATION
Amiodarone and Simvastatin Legal News Articles & Interviews
FDA Announces Safety Changes for Simvastatin
 June 8, 2011. By Heidi Turner.
June 8, 2011. By Heidi Turner.Scientists Discover Previously Unknown Simvastatin Effect
 February 21, 2011. By Charles Benson.
February 21, 2011. By Charles Benson.Amiodarone or Multaq: Where Should Patients Turn?
 February 11, 2011. By Heidi Turner.
February 11, 2011. By Heidi Turner.French Company to Warn US Doctors of Side Effects of Drug Similar to Amiodarone
 January 22, 2011. By Charles Benson.
January 22, 2011. By Charles Benson.Amiodarone: When Off-Label Drug Use Hurts Patients
 January 9, 2011. By Heidi Turner.
January 9, 2011. By Heidi Turner.Amiodarone: Father Went into Hospital and Never Came Out
 January 4, 2011. By Heidi Turner.
January 4, 2011. By Heidi Turner.Amiodarone Interaction with Simvastatin Still a Global Concern
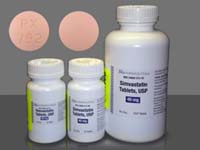 December 20, 2010. By Gordon Gibb.
December 20, 2010. By Gordon Gibb.Amiodarone and Simvastatin Anything But a Dynamic Duo
 November 19, 2009. By Gordon Gibb.
November 19, 2009. By Gordon Gibb.Off-label use of Amiodarone may be dangerous
 November 4, 2009. By Charles Benson.
November 4, 2009. By Charles Benson.Amiodarone + Simvastatin = Increased Risk: FDA
 October 18, 2009. By Gordon Gibb.
October 18, 2009. By Gordon Gibb.- FDA: Simvastatin and Amiodarone Don't Mix By Gordon Gibb (Aug-9-08)
