LAWSUITS NEWS & LEGAL INFORMATION
Multaq Legal News Articles & Interviews
A Year Has Passed Since Trial Halted Due to Multaq Side Effects
 August 21, 2012. By Gordon Gibb.
August 21, 2012. By Gordon Gibb.Weighing the Pros and Cons of Multaq
 July 13, 2012. By Heidi Turner.
July 13, 2012. By Heidi Turner.Multaq Recommended As a Second- or Third-Line Treatment
 June 28, 2012. By Heidi Turner.
June 28, 2012. By Heidi Turner."Multaq Not a Very Safe Drug"
 May 16, 2012. By Gordon Gibb.
May 16, 2012. By Gordon Gibb.Is Multaq Worth the Risks?
 April 14, 2012. By Heidi Turner.
April 14, 2012. By Heidi Turner.Multaq Concerns Reverberate through the Cardiology Community
 March 27, 2012. By Gordon Gibb.
March 27, 2012. By Gordon Gibb.Concerns about Multaq Side Effects
 February 21, 2012. By Heidi Turner.
February 21, 2012. By Heidi Turner.In February, the Message on Heart Disease Is Not Complete
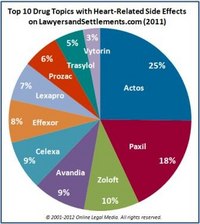 February 2, 2012. By Gordon Gibb.
February 2, 2012. By Gordon Gibb.Multaq Hit with Second Black Box Warning in As Many Years
 January 19, 2012. By Gordon Gibb.
January 19, 2012. By Gordon Gibb.FDA Review of Multaq Side Effects
 November 1, 2011. By Heidi Turner.
November 1, 2011. By Heidi Turner.