LAWSUITS NEWS & LEGAL INFORMATION
Alere INRatio Recall Legal News Articles & Interviews
Merger Deal between Device Manufacturers Reveals Device Shortcomings
 June 6, 2017. By Gordon Gibb.
June 6, 2017. By Gordon Gibb.Waltham, MA: A merger originally gone bad between two medical device manufacturers, since rescued, sheds some light into previous problems Alere Inc. has had with its measuring devices and, specifically the INRatio blood monitoring system. Various plaintiffs embroiled in an INRatio lawsuit allege the expensive monitoring device was inaccurate, flawed and not safe for all patients.
Read [ Merger Deal between Device Manufacturers Reveals Device Shortcomings ]
INRatio Proposed Class Action Settled Before Alere Lawsuit Could Proceed
 May 1, 2017. By Gordon Gibb.
May 1, 2017. By Gordon Gibb.Waltham, MA: An Alere lawsuit that had been proposed as a class action on behalf of litigants alleging injury from inaccurate readings from the INRatio blood monitoring device escaped trial when the parties involved agreed to a settlement at the eleventh hour. Briefings related to J.E. et al. v. Alere Inc. et al (Case No. 1:16-cv-11515, in the US District Court for the District of Massachusetts), and motions of dismissal put forward by Alere Inc. had been duly submitted, but the judge in the case had yet to rule on them when the settlement was announced.
Read [ INRatio Proposed Class Action Settled Before Alere Lawsuit Could Proceed ]
Alere Pushes for Dismissal of Home Blood Test Kits Class Action
 March 19, 2017. By Deb Hipp.
March 19, 2017. By Deb Hipp.Boston, MA: In response to plaintiffs' January motion to keep an Alere INRatio class action lawsuit alive, Alere recently pushed back with arguments that the court should dismiss the case.
Read [ Alere Pushes for Dismissal of Home Blood Test Kits Class Action ]
Failed INRatio Device Fast-Tracked to Market
 March 10, 2017. By Gordon Gibb.
March 10, 2017. By Gordon Gibb.San Diego, CA: A new Alere lawsuit filed earlier this month in San Diego asserts that the allegedly failed Alere INRatio measuring device was approved through the issuance of a 510(k) Clearance by the US Food and Drug Administration (FDA). The problematic measuring system, comprised of test strips and a measuring device, were subject to an FDA-sanctioned Alere recall in 2014. Lawsuits are continuing to roll in.
Read [ Failed INRatio Device Fast-Tracked to Market ]
Alere INRatio Plaintiffs Petition Court to Keep Lawsuit Alive
 February 17, 2017. By Gordon Gibb.
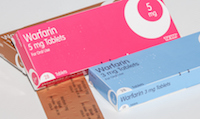
February 17, 2017. By Gordon Gibb.Boston, MA: The INRatio measuring system manufactured by Alere Inc. (Alere) and subject to an Alere recall last year, has oft been highlighted as part of a study focused on the effectiveness of a new-generation blood thinner when compared against warfarin. The oft-made allegation is that inaccurate readings may have given the newer anticoagulant, in this case Xarelto, an unfair advantage over Coumadin (warfarin). The US Food and Drug Administration (FDA) has since opined that in its view, the variances in readings were not drastic enough to sufficiently skew the results.
Read [ Alere INRatio Plaintiffs Petition Court to Keep Lawsuit Alive ]
INRatio Class Action Headed for Trial
 February 7, 2017. By Brenda Craig.
February 7, 2017. By Brenda Craig.Los Angeles, CA The Alere INRatio home monitoring system offered warfarin (Coumadin) users a convenient way to test their blood clotting rate without having to go the doctor’s office or a laboratory. Instead, they got an expensive, unreliable and potentially dangerous product.
Read [ INRatio Class Action Headed for Trial ]
Adverse Events for Patients Using Alere INRatio System
 January 21, 2017. By Heidi Turner.
January 21, 2017. By Heidi Turner.Houston, TX: Patients taking blood thinning medications know they have to be careful to ensure their blood remains in therapeutic levels, to prevent major bleeding episodes. That’s why they use devices like the Alere INRatio2 system, to ensure that if they need it, their blood clots properly. But what happens if they can't be sure the systems that are meant to ensure their safety are actually working properly? That's the issue that was faced by some patients who use the Alere InRatio system, after the system was recalled due to concerns regarding inaccurate results.
Read [ Adverse Events for Patients Using Alere INRatio System ]
Alere Inc. In Tough over Recalled INRatio, Abbott Merger in Jeopardy
 January 3, 2017. By Gordon Gibb.
January 3, 2017. By Gordon Gibb.Georgetown, DE: An Alere lawsuit filed by former suitor Abbott Laboratories Inc. (Abbott) over a proposed merger is, for the moment, redirecting attention away from the failed INRatio measuring device that was recalled in July. The recalled product nonetheless has a role in the recent performance of Alere Inc. (Alere) that has prompted Abbott to back away. This, in spite of recent assurances from the US Food and Drug Administration (FDA) that any failings of the Alere INRatio device had no bearing on the relationship between warfarin (Coumadin) and Xarelto (rivaroxaban), and the subsequent approval of the latter.
Read [ Alere Inc. In Tough over Recalled INRatio, Abbott Merger in Jeopardy ]
Three Points for Alere INRatio Device
 December 21, 2016. By Jane Mundy.
December 21, 2016. By Jane Mundy.Dallas, TX: Negligence allegations against Janssen Pharmaceuticals, Johnson & Johnson and Bayer AG may have fizzled somewhat. Two recent issues do not bode well for plaintiffs filing Alere INRatio lawsuits. The FDA recently announced that the Alere INRatio anti-coagulation monitoring device had no substantial effect on the outcome of the ROCKET-AF trial and in turn did not significantly favor Xarelto as earlier suspected, and a California Alere class action suit in September was dismissed.
Read [ Three Points for Alere INRatio Device ]
FDA Weighs in Alere Recall and Potential for Skewed Results
 December 3, 2016. By Gordon Gibb.
December 3, 2016. By Gordon Gibb.Washington, DC: On the heels of a published report in BMJ with regard to the Alere INRatio device, the Alere Recall
Read [ FDA Weighs in Alere Recall and Potential for Skewed Results ]
- Janssen Knew About Issues With Alere INRatio Device By Heidi Turner (Oct-6-16)
- Alere Inaccuracies may lead to Legal Action By Jane Mundy (Sep-24-16)
