LAWSUITS NEWS & LEGAL INFORMATION
Zimmer Biomet Recalls Comprehensive Reverse Shoulder Replacement
Were you looking for Zimmer Biomet Shoulder Recall lawsuits?
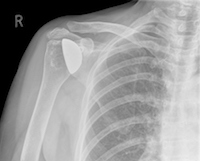
Washington, DC: The US Food and Drug Administration (FDA) has issued notification that Zimmer Biomet is recalling the Comprehensive Reverse Shoulder because these devices are fracturing at a higher rate than is stated in the labeling. Fractures may result in revision surgeries which could cause serious adverse health consequences such as permanent loss of shoulder function, infection, or rarely, death.
The Comprehensive Reverse Shoulder is a shoulder replacement device that is surgically implanted to help restore arm movement. This device is beneficial for patients with rotator cuff tears who have developed a severe type of shoulder arthritis known as arthropathy and previously failed shoulder joint replacement.
On December 20, 2016 Zimmer Biomet sent an Urgent Medical Device Recall Notice and a Certificate of Acknowledgement form to all affected customers. The notice also stated that there are no specific patient monitoring instructions related to this recall that are recommended beyond existing surgical follow up protocol.
Last updated on
The Comprehensive Reverse Shoulder is a shoulder replacement device that is surgically implanted to help restore arm movement. This device is beneficial for patients with rotator cuff tears who have developed a severe type of shoulder arthritis known as arthropathy and previously failed shoulder joint replacement.
On December 20, 2016 Zimmer Biomet sent an Urgent Medical Device Recall Notice and a Certificate of Acknowledgement form to all affected customers. The notice also stated that there are no specific patient monitoring instructions related to this recall that are recommended beyond existing surgical follow up protocol.
Legal Help
If you or a loved one has suffered similar damages or injuries, please fill in our form and your complaint will be sent to a lawyer who may evaluate your claim at no cost or obligation.Last updated on
DEFECTIVE PRODUCTS LEGAL ARTICLES AND INTERVIEWS
Zimmer Biomet Shoulder Failure: Consider Rehabilitation over Revision Surgery
Zimmer-Biomet Shoulder: Buyer (Patient and Surgeon) Beware
Zimmer Biomet Shoulder—Shocking Surprises

June 27, 2017
Washington, DC: Not only is the failure rate of Zimmer Biomet shoulder surgery abnormally high, a new study shows that some patients undergoing shoulder replacement surgery have an increased risk of complications, including the need for revision surgery—which comes with its own set of risks. READ MORE
Zimmer-Biomet Shoulder: Buyer (Patient and Surgeon) Beware

May 25, 2017
Minneapolis, MN: Shoulder replacement surgery is a well-established and should be a straightforward procedure, unless your orthopedic surgeon is implanting a Zimmer Biomet shoulder that has failed and you are unfortunate to have one or two of the 3,600 implants that have been recalled. READ MORE
Zimmer Biomet Shoulder—Shocking Surprises

April 22, 2017
Washington, DC: The fact that Zimmer Biomet had its Comprehensive Reverse Shoulder approved without any human testing likely comes as a shocking surprise to many recipients of the device. And another surprise: the device was pulled from the market in 2010 after Biomet received complaints of fracturing. But it returned just one year later… READ MORE
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
