LAWSUITS NEWS & LEGAL INFORMATION
Oral Sodium Phosphate (OSP) Kidney Failure
In December 2008, the FDA announced that the use of oral sodium phosphate products, including Fleet Phospho-soda, and prescription-only Visicol and OsmoPrep, is associated with acute phosphate nephropathy, an acute kidney failure. The oral sodium phosphate products in question are used for bowel cleansing prior to colonoscopy and other procedures. The FDA now requires that oral sodium phosphate products available by prescription--Visicol and OsmoPrep--carry a boxed warning. Meanwhile, the maker of Fleet Phospho-soda, an over-the-counter oral sodium phosphate product, has announced a recall of that product.
FREE ORAL SODIUM PHOSPHATE LAWSUIT EVALUATION
Send your Oral Sodium Phosphate claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Oral Sodium Phosphate Kidney Failure
The FDA has announced that a boxed warning will be added to oral sodium phosphate products, warning of the risk of acute phosphate nephropathy—an acute kidney injury. The announcement comes after more than 20 reports of the condition were linked to the use of oral sodium phosphates. The products receiving the boxed warning are prescription products Visicol and OsmoPrep, which are used for bowel cleansing prior to colonoscopy and other procedures. The FDA has also recommended that another product, Fleet Phospho-soda, should only be used as a laxative and not as a bowel cleanser.
 The FDA reports that of the 20 cases of acute phosphate nephropathy associated with the oral sodium phosphates, five cases were reportedly life-threatening and 10 resulted in hospitalization. Furthermore, four patients required dialysis and one patient died from complications of pneumonia. The onset of acute phosphate nephropathy was between seven hours and 21 days following the use of the oral sodium phosphate.
The FDA reports that of the 20 cases of acute phosphate nephropathy associated with the oral sodium phosphates, five cases were reportedly life-threatening and 10 resulted in hospitalization. Furthermore, four patients required dialysis and one patient died from complications of pneumonia. The onset of acute phosphate nephropathy was between seven hours and 21 days following the use of the oral sodium phosphate.
Death has allegedly been reported in cases where patients took sodium phosphate products and suffered fluid shifts, electrolyte abnormalities and heart problems. Other reported adverse reactions include atrial fibrillation and abnormal heart rhythm associated with Visicol. A 2002 FDA news alert reports that Visicol was associated with patients experiencing seizures. Additional side effects associated with oral sodium phosphate products include nausea, vomiting and abdominal pain.
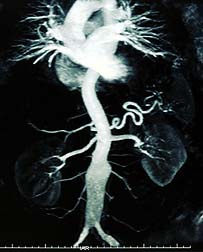
According to the FDA, acute phosphate nephropathy is "a form of acute kidney injury that is associated with deposits of calcium-phosphate crystals in the renal tubules that may result in permanent renal function impairment." It is a rare and serious adverse event.
People at highest risk of acute phosphate nephropathy are those over the age of 55, those who are hypervolemic or suffer from decreased intravascular volume, those who have baseline kidney disease, bowel obstruction or active colitis, and those who are taking medications that affect renal function, such as diuretics. Patients with impaired kidney function may be more likely to suffer adverse reactions because sodium phosphate is reportedly excreted through the kidneys.
Fleet Phospho-Soda, Visicol and OsmoPrep
In addition to a boxed warning, the makers of Visicol and OsmoPrep have been required to develop and implement a risk evaluation and mitigation strategy to ensure that the benefits of those products outweigh the risks.
Meanwhile, CB Fleet, maker of the Fleet Phospho-soda and the Fleet Phospho-soda EZ-Prep Bowel Cleansing System, has voluntarily recalled those two products. The FDA has recommended that those products not be used for bowel cleansing. They were previously sold as over-the-counter products to cleanse bowels prior to colonoscopy and other procedures. Fleet Phospho-soda is known generically as sodium biphosphate and sodium phosphate.
Visicol, marketed by Salix Pharmaceuticals, is a tablet given to patients before a colonoscopy and is designed to clear the patient's bowels and colon. Patients are required to take more than 40 tablets in a 24-hour period to induce diarrhea. A colonoscopy is used to help diagnose colon cancer, inflammatory bowel disease (IBD), anemia and polyps. The procedure involves an in-depth examination of the patient's large intestine using a fiber optic camera.
OsmoPrep, also marketed by Salix Pharmaceuticals, is a prescription tablet taken to clear a patient's bowels and colon prior to a colonoscopy. With OsmoPrep, the patient is required to take a series of tablets--32 in total--as directed during the 24-hour period prior to the colonoscopy procedure. Because both OsmoPrep and Visicol are tablets taken orally, patients are required to drink ample amounts of clear fluids along with the prescription in order to remain adequately hydrated.
 The FDA reports that of the 20 cases of acute phosphate nephropathy associated with the oral sodium phosphates, five cases were reportedly life-threatening and 10 resulted in hospitalization. Furthermore, four patients required dialysis and one patient died from complications of pneumonia. The onset of acute phosphate nephropathy was between seven hours and 21 days following the use of the oral sodium phosphate.
The FDA reports that of the 20 cases of acute phosphate nephropathy associated with the oral sodium phosphates, five cases were reportedly life-threatening and 10 resulted in hospitalization. Furthermore, four patients required dialysis and one patient died from complications of pneumonia. The onset of acute phosphate nephropathy was between seven hours and 21 days following the use of the oral sodium phosphate.
Death has allegedly been reported in cases where patients took sodium phosphate products and suffered fluid shifts, electrolyte abnormalities and heart problems. Other reported adverse reactions include atrial fibrillation and abnormal heart rhythm associated with Visicol. A 2002 FDA news alert reports that Visicol was associated with patients experiencing seizures. Additional side effects associated with oral sodium phosphate products include nausea, vomiting and abdominal pain.
According to the FDA, acute phosphate nephropathy is "a form of acute kidney injury that is associated with deposits of calcium-phosphate crystals in the renal tubules that may result in permanent renal function impairment." It is a rare and serious adverse event.
People at highest risk of acute phosphate nephropathy are those over the age of 55, those who are hypervolemic or suffer from decreased intravascular volume, those who have baseline kidney disease, bowel obstruction or active colitis, and those who are taking medications that affect renal function, such as diuretics. Patients with impaired kidney function may be more likely to suffer adverse reactions because sodium phosphate is reportedly excreted through the kidneys.
Fleet Phospho-Soda, Visicol and OsmoPrep
In addition to a boxed warning, the makers of Visicol and OsmoPrep have been required to develop and implement a risk evaluation and mitigation strategy to ensure that the benefits of those products outweigh the risks.
Meanwhile, CB Fleet, maker of the Fleet Phospho-soda and the Fleet Phospho-soda EZ-Prep Bowel Cleansing System, has voluntarily recalled those two products. The FDA has recommended that those products not be used for bowel cleansing. They were previously sold as over-the-counter products to cleanse bowels prior to colonoscopy and other procedures. Fleet Phospho-soda is known generically as sodium biphosphate and sodium phosphate.
Visicol, marketed by Salix Pharmaceuticals, is a tablet given to patients before a colonoscopy and is designed to clear the patient's bowels and colon. Patients are required to take more than 40 tablets in a 24-hour period to induce diarrhea. A colonoscopy is used to help diagnose colon cancer, inflammatory bowel disease (IBD), anemia and polyps. The procedure involves an in-depth examination of the patient's large intestine using a fiber optic camera.
OsmoPrep, also marketed by Salix Pharmaceuticals, is a prescription tablet taken to clear a patient's bowels and colon prior to a colonoscopy. With OsmoPrep, the patient is required to take a series of tablets--32 in total--as directed during the 24-hour period prior to the colonoscopy procedure. Because both OsmoPrep and Visicol are tablets taken orally, patients are required to drink ample amounts of clear fluids along with the prescription in order to remain adequately hydrated.
Oral Sodium Phosphate (OSP) Kidney Failure Legal Help
If you or a loved one has suffered damages in this case, please click the link below and your complaint will be sent to a lawyer who may evaluate your claim at no cost or obligation.Last updated on
ORAL SODIUM PHOSPHATE LEGAL ARTICLES AND INTERVIEWS
OSP Manufacturer Salix Sued, Alleged Kidney Damage from OsmoPrep
Risks of OSP and Kidneys Articulated One Year Before FDA Alert
OSP Kidney Failure Lawsuits Still Coming


February 27, 2010
The US Food and Drug Administration (FDA) recently weighed in on oral sodium phosphates (OSP) and acute phosphate nephropathy after receiving more than 20 reports of kidney damage linked to the use of oral sodium phosphates. READ MORE
Risks of OSP and Kidneys Articulated One Year Before FDA Alert

November 11, 2009
A group that knows a lot about oral sodium phosphate and OSP kidney damage recently wrapped up its 42nd annual conference in San Diego. The yearly meeting and scientific exposition, dubbed "Renal Week" by the American Society of Nephrology (ASN), took place from October 27th to November 1st. READ MORE
OSP Kidney Failure Lawsuits Still Coming

October 13, 2009
Sometimes it's difficult to talk about the nether regions of the human body, but the fact remains that the public must be notified: a preparation used to cleanse the bowel prior to colonoscopy is suspected of having the potential to trigger such dire consequences as OSP kidney damage. OSP is shorthand for oral sodium phosphate. One product—Phospho-soda and manufactured by Fleet—has been flagged by the US Food and Drug Administration (FDA). READ MORE
READ MORE Drugs/Medical Settlements and Legal News
