LAWSUITS NEWS & LEGAL INFORMATION
Duodenoscope Infection Risk
Were you looking for CRE Superbug Outbreak at UCLA Ronald Reagan Medical Center or FDA Warns of Infection Risk with Duodenoscope or Infection Risk With Reprocessed Flexible Bronchoscopes lawsuits?
By Heidi Turner
The US Food and Drug Administration (FDA) has issued a warning that duodenoscopes could be linked to the spread of deadly bacteria. This warning concerns ERCP endoscopes, also known as duodenoscopes, and comes on the heels of reports of serious multi-drug resistant bacteria in patients who had medical procedures involving the medical devices. Duodenoscope lawsuits have now been filed after patients reportedly developed infections linked to devices.
 On February 19, 2015, the FDA warned that the complex design of ERCP endoscopes may prevent them from being properly sanitized, even when manufacturer recommended sanitation guidelines are followed.
On February 19, 2015, the FDA warned that the complex design of ERCP endoscopes may prevent them from being properly sanitized, even when manufacturer recommended sanitation guidelines are followed.
"Meticulously cleaning duodenoscopes prior to high-level disinfection should reduce the risk of transmitting infection, but may not entirely eliminate it," the FDA warned.
The agency noted that from January 2013 to December 2014 it received 75 medical device reports involving around 135 patients who suffered a possible microbial transmission from duodenoscopes. The FDA said it will continue to monitor the association between duodenoscopes and infectious agents.
One medical center linked to the outbreak is Ronald Reagan Medical Center at UCLA. The medical center said seven patients were infected by a bacteria linked to duodenoscopes. Two people reportedly died and 179 others may have been exposed, according to the Los Angeles Times (2/25/15).
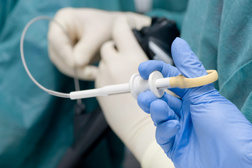 Duodenoscopes are lighted tubes inserted in a patient through the mouth to the top of the small intestine. They are used during endoscopic retrograde cholangiopancreatography (ERCP), a procedure involved in diagnosing and treating problems of the pancreas and bile ducts.
Duodenoscopes are lighted tubes inserted in a patient through the mouth to the top of the small intestine. They are used during endoscopic retrograde cholangiopancreatography (ERCP), a procedure involved in diagnosing and treating problems of the pancreas and bile ducts.
Because the duodenoscopes have many small parts, tissue or fluid from one patient can remain on a duodenoscope even after it is cleaned. This can result in a patient-to-patient infection. The FDA has said that the infections have occurred even after medical professionals followed proper manufacturer cleaning instructions.
An 18-year-old who developed a CRE (carbapenem-resistant Enterobacteriaceae) infection after undergoing a procedure involving a duodenoscope has filed a lawsuit against Olympus, maker of the device. According to the Los Angeles Times, although Olympus redesigned its duodenoscope it did not provide proper cleaning guidelines for the product.
Last updated on
FREE DUODENOSCOPE INFECTION LAWSUIT EVALUATION
Send your Duodenoscope Infection claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Duodenoscope Super Bug
 On February 19, 2015, the FDA warned that the complex design of ERCP endoscopes may prevent them from being properly sanitized, even when manufacturer recommended sanitation guidelines are followed.
On February 19, 2015, the FDA warned that the complex design of ERCP endoscopes may prevent them from being properly sanitized, even when manufacturer recommended sanitation guidelines are followed. "Meticulously cleaning duodenoscopes prior to high-level disinfection should reduce the risk of transmitting infection, but may not entirely eliminate it," the FDA warned.
The agency noted that from January 2013 to December 2014 it received 75 medical device reports involving around 135 patients who suffered a possible microbial transmission from duodenoscopes. The FDA said it will continue to monitor the association between duodenoscopes and infectious agents.
One medical center linked to the outbreak is Ronald Reagan Medical Center at UCLA. The medical center said seven patients were infected by a bacteria linked to duodenoscopes. Two people reportedly died and 179 others may have been exposed, according to the Los Angeles Times (2/25/15).
Duodenoscopes
 Duodenoscopes are lighted tubes inserted in a patient through the mouth to the top of the small intestine. They are used during endoscopic retrograde cholangiopancreatography (ERCP), a procedure involved in diagnosing and treating problems of the pancreas and bile ducts.
Duodenoscopes are lighted tubes inserted in a patient through the mouth to the top of the small intestine. They are used during endoscopic retrograde cholangiopancreatography (ERCP), a procedure involved in diagnosing and treating problems of the pancreas and bile ducts. Because the duodenoscopes have many small parts, tissue or fluid from one patient can remain on a duodenoscope even after it is cleaned. This can result in a patient-to-patient infection. The FDA has said that the infections have occurred even after medical professionals followed proper manufacturer cleaning instructions.
Duodenoscope Lawsuits
Duodenoscope Infection Legal Help
If you or a loved one has suffered similar damages or injuries, please click the link below and your complaint will be sent to a lawyer who may evaluate your claim at no cost or obligation.Last updated on
DUODENOSCOPE INFECTION LEGAL ARTICLES AND INTERVIEWS
ED-530XT Duodenoscopes Recalled
Older Model Duodenoscopes Pulled from Market

Olympus Failed to Warn US Hospital of Duodenoscope Infections


July 21, 2017
Santa Clara, CA: An urgent recall of all Fujifilm ED-530XT duodenoscopes has been issued, due to the difficulty in what the US Food and Drug Administration (FDA) refers to as “reprocessing.” Reprocessing is a detailed, multistep process to clean and disinfect or sterilize reusable devices. The voluntary recall of the duodenoscopes also includes replacement of the ED-530XT forceps elevator mechanism including the O-ring seal, replacement of the distal end cap, and new Operation Manuals. The FDA has previously issued a warning that duodenoscopes could be linked to the spread of deadly bacteria. READ MORE
Older Model Duodenoscopes Pulled from Market

January 13, 2017
Santa Clara, CA: Fuji, the maker of allegedly defective duodenoscopes, has announced it plans to remove its legacy 250/450 duodenoscope models from clinical use. The decision is reportedly based on the limited number of duodenoscopes currently in use, not because of a known safety risk, and noted that it has not received any recent reports of adverse events associated with these scopes. READ MORE
Olympus Failed to Warn US Hospital of Duodenoscope Infections

July 26, 2016
Santa Clara, CA: According to news reports from CNBC and the LA Times, contaminated Olympus duodenoscopes READ MORE
READ MORE Operating Room Equipment Settlements and Legal News
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
