LAWSUITS NEWS & LEGAL INFORMATION
Asthma Medications Side Effects
In February, 2010, the US Food and Drug Administration (FDA) issued a warning about the risk of certain Asthma inhaler side effects. There is now a Serevent and an Advair black box warning, alerting patients to the risk of using those medications. Furthermore, patients are warned to be on the alert for Foradil side effects, which can include worsened breathing. The FDA warns that patients using a certain class of asthma drugs—Long Acting Beta Agonists—should follow specific recommendations to reduce the risks of adverse effects.
Medications used to treat asthma include Serevent (salmeterol), Foradil (formoterol), Symbicort (combination of budesonide and formorterol) and Advair (combination of fluticasone and salmeterol).
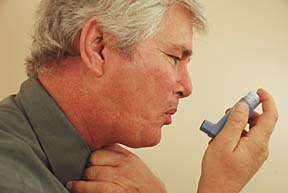 According to the American Lung Association, approximately 23 million Americans have asthma, chronic inflammatory lung disease in which airways are blocked or narrowed and breathing becomes difficult. Allergic asthma occurs when the symptoms—wheezing, breathing difficulties and coughing—are caused by an allergic reaction to an inhaled allergen. Non-allergic asthma occurs when the symptoms of asthma are caused by an inhaled irritant.
According to the American Lung Association, approximately 23 million Americans have asthma, chronic inflammatory lung disease in which airways are blocked or narrowed and breathing becomes difficult. Allergic asthma occurs when the symptoms—wheezing, breathing difficulties and coughing—are caused by an allergic reaction to an inhaled allergen. Non-allergic asthma occurs when the symptoms of asthma are caused by an inhaled irritant.
There is no cure for asthma. Patients with asthma can only hope to control it, either through preventive medicines or quick relievers. Preventive medicines are used for long-term asthma control and to decrease the number and severity of asthma attacks. Quick reliever medicines are used in the case of an asthma attack to relieve the symptoms.
Drugs known as Long Acting Beta Agonists (LABAs) are often used for long-term asthma control. They work by relaxing the muscles in the patient's airway and lungs, allowing the patient to breathe easier. Serevent and Foradil are LABA-only asthma medications, while Advair and Symbicort contain LABAs and inhaled corticosteroids.
FDA Warning
In February, 2010, the FDA issued a warning concerning the use of LABAs. The FDA's warning urged physicians to move patients away from the use of medicines that contained both LABAs and inhaled corticosteroids. Furthermore, the FDA recommended that patients whose asthma is only controlled by the use of LABAs should use the LABAs for the shortest time possible and should only use the LABAs in combination with inhaled corticosteroids.
As a result of the FDA's warning, manufacturers will have to include new warnings on the drugs' labels about the risk associated with the use of LABAs. Furthermore, the companies will have to study whether the use of LABAs with other drugs enhances their safety.
The FDA announced the warning after clinical trial data suggested a link between the use of LABAs and an increased risk of severe exacerbation of asthma symptoms that lead to hospitalizations and, in certain cases, death.
Asthma Medications and Asthma-Related Deaths
Advair and Serevent both include black box warnings alerting patients to the increased risk of asthma-related deaths in patients who receive salmeterol. According to the warning label, the risk of death for patients who received salmeterol is 13 deaths out of 13,176 patients compared with 3 out of 13,179 patients who received a placebo.
A US FDA advisory panel voted unanimously that Novartis' Foradil asthma medication, marketed by Schering-Plough Corp., should have a warning regarding the risk for worsened breathing. Furthermore, Foradil's label includes a warning that Foradil Aerolizer should only be used for patients whose asthma is not adequately controlled by other medications or whose disease severity clearly warrants treatment with Foradil Aerolizer. The warning also notes that the results of the salmeterol studies may also apply to formoterol.
Recent studies have found that patients who inhaled the long-acting beta-agonists Serevent and Advair (salmeterol) or Foradil (formoterol), were 3.5 times more likely to die from asthma. Apparently, while these drugs relieve asthma symptoms, the medication also creates bronchial inflammation, which can lead to death.
Serevent and Advair manufactured by GlaxoSmithKline. Schering Corporation, which is a subsidiary of Merck & Co., Inc., is the exclusive U.S. marketer and distributor of Foradil, which is manufactured by Novartis.
According to astrophysicist Edwin Salpeter "We can show that overall it is statistically significant that, compared to patients taking a placebo, these long-acting beta-agonists kill a lot of people."
Shelley Salpeter, clinical professor of medicine at Stanford's School of Medicine confirms: "We estimate that approximately 4,000 out of the 5,000 asthma deaths that occur in the U.S. each year are actually caused by these long-acting beta-agonists, and we urge that these agents be taken off the market."
A study conducted by Shelley Salpeter and published in the Annals of Internal Medicine (06/20/06) concluded, "[Long-lasting Beta-Agonists] have been shown to increase severe and life-threatening asthma exacerbations, as well as asthma-related deaths."
And more tragic, most of these asthma deaths are otherwise healthy young adults.
Last updated on
FREE ASTHMA DRUGS LAWSUIT EVALUATION
Send your Asthma Drugs claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Asthma Drug Side Effects
 According to the American Lung Association, approximately 23 million Americans have asthma, chronic inflammatory lung disease in which airways are blocked or narrowed and breathing becomes difficult. Allergic asthma occurs when the symptoms—wheezing, breathing difficulties and coughing—are caused by an allergic reaction to an inhaled allergen. Non-allergic asthma occurs when the symptoms of asthma are caused by an inhaled irritant.
According to the American Lung Association, approximately 23 million Americans have asthma, chronic inflammatory lung disease in which airways are blocked or narrowed and breathing becomes difficult. Allergic asthma occurs when the symptoms—wheezing, breathing difficulties and coughing—are caused by an allergic reaction to an inhaled allergen. Non-allergic asthma occurs when the symptoms of asthma are caused by an inhaled irritant.
There is no cure for asthma. Patients with asthma can only hope to control it, either through preventive medicines or quick relievers. Preventive medicines are used for long-term asthma control and to decrease the number and severity of asthma attacks. Quick reliever medicines are used in the case of an asthma attack to relieve the symptoms.
Drugs known as Long Acting Beta Agonists (LABAs) are often used for long-term asthma control. They work by relaxing the muscles in the patient's airway and lungs, allowing the patient to breathe easier. Serevent and Foradil are LABA-only asthma medications, while Advair and Symbicort contain LABAs and inhaled corticosteroids.
FDA Warning
In February, 2010, the FDA issued a warning concerning the use of LABAs. The FDA's warning urged physicians to move patients away from the use of medicines that contained both LABAs and inhaled corticosteroids. Furthermore, the FDA recommended that patients whose asthma is only controlled by the use of LABAs should use the LABAs for the shortest time possible and should only use the LABAs in combination with inhaled corticosteroids.
As a result of the FDA's warning, manufacturers will have to include new warnings on the drugs' labels about the risk associated with the use of LABAs. Furthermore, the companies will have to study whether the use of LABAs with other drugs enhances their safety.
The FDA announced the warning after clinical trial data suggested a link between the use of LABAs and an increased risk of severe exacerbation of asthma symptoms that lead to hospitalizations and, in certain cases, death.
Asthma Medications and Asthma-Related Deaths
Advair and Serevent both include black box warnings alerting patients to the increased risk of asthma-related deaths in patients who receive salmeterol. According to the warning label, the risk of death for patients who received salmeterol is 13 deaths out of 13,176 patients compared with 3 out of 13,179 patients who received a placebo.
A US FDA advisory panel voted unanimously that Novartis' Foradil asthma medication, marketed by Schering-Plough Corp., should have a warning regarding the risk for worsened breathing. Furthermore, Foradil's label includes a warning that Foradil Aerolizer should only be used for patients whose asthma is not adequately controlled by other medications or whose disease severity clearly warrants treatment with Foradil Aerolizer. The warning also notes that the results of the salmeterol studies may also apply to formoterol.
Recent studies have found that patients who inhaled the long-acting beta-agonists Serevent and Advair (salmeterol) or Foradil (formoterol), were 3.5 times more likely to die from asthma. Apparently, while these drugs relieve asthma symptoms, the medication also creates bronchial inflammation, which can lead to death.
Serevent and Advair manufactured by GlaxoSmithKline. Schering Corporation, which is a subsidiary of Merck & Co., Inc., is the exclusive U.S. marketer and distributor of Foradil, which is manufactured by Novartis.
According to astrophysicist Edwin Salpeter "We can show that overall it is statistically significant that, compared to patients taking a placebo, these long-acting beta-agonists kill a lot of people."
Shelley Salpeter, clinical professor of medicine at Stanford's School of Medicine confirms: "We estimate that approximately 4,000 out of the 5,000 asthma deaths that occur in the U.S. each year are actually caused by these long-acting beta-agonists, and we urge that these agents be taken off the market."
A study conducted by Shelley Salpeter and published in the Annals of Internal Medicine (06/20/06) concluded, "[Long-lasting Beta-Agonists] have been shown to increase severe and life-threatening asthma exacerbations, as well as asthma-related deaths."
And more tragic, most of these asthma deaths are otherwise healthy young adults.
Asthma Drugs Side Effects Legal Help
If a loved one has died while using Serevent, Advair, or Foradil, you may qualify for damages or remedies that may be awarded in a possible lawsuit. Please click the link below to send your complaint to a lawyer who will review your claim at no cost or obligation.Last updated on
ASTHMA DRUGS LEGAL ARTICLES AND INTERVIEWS
Newly Discovered Cell Could Help Avoid Asthma Medication Side Effects

Safety Requirements for Advair and Other Asthma Medications Changed

A Near Fatal Asthma Attack Possibly Caused by Serevent

March 26, 2010
Parents concerned about asthma medication side effects in their children may be in luck, as a newly discovered white blood cell has shown potential as a treatment for the bronchial disorder. READ MORE
Safety Requirements for Advair and Other Asthma Medications Changed

February 20, 2010
The FDA has announced that it is changing the safety requirements for asthma medications, including Advair. The affected medications are Long-Acting Beta-Agonists (LABAs) sold as single-ingredient products (Serevent and Foradil) and as an ingredient in products that contain inhaled corticosteroids (Advair and Symbicort). These medications are used to treat asthma and chronic obstructive pulmonary disease (COPD). READ MORE
A Near Fatal Asthma Attack Possibly Caused by Serevent

June 18, 2008
Mary is asthmatic. She had been taking Serevent for about 18 months when she had a near-fatal acute asthma attack. Her regular inhaler didn't help, where normally it would have. She started losing her breath, and her ability to speak. Her husband called 911 and she was rushed to the emergency room at the nearest hospital. READ MORE
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
lynn
on
New Jersey
on