 It was 13 years ago, on August 6th 1996 when Public Citizen first petitioned the US Food and Drug Administration (FDA) to place warnings with regard to tendonitis and tendon rupture on the package inserts of all fluoroquinolone-classed drugs. Cipro is one of them.
It was 13 years ago, on August 6th 1996 when Public Citizen first petitioned the US Food and Drug Administration (FDA) to place warnings with regard to tendonitis and tendon rupture on the package inserts of all fluoroquinolone-classed drugs. Cipro is one of them.The warning was indeed placed. However a non-bolded warning buried in the list of possible adverse reactions proved ineffective (according to Public Citizen) in educating consumers as to the risks associated with taking the Cipro antibiotic.
And so, Public Citizen was back again—in August 2006—this time petitioning the FDA to implement a black box warning for Cipro.
It backed up its request with research. According to the official Public Citizen website the advocacy group searched the FDA Adverse Event database spanning November 1997 through the end of 2005. It found enough evidence of tendon rupture and various adverse reactions to warrant a petition to the FDA with regard to the addition of a black box warning.
Nor was Public Citizen the only petitioner. A year prior, on May 28, 2005 Dr. Arnold Widen, Medical Director of the Illinois Attorney General's Office and Dr. Babs Waldman, Medical Director of the Health Care Bureau of the Attorney General's Office for Illinois petitioned the FDA to issue a black box warning over tendon rupture for Cipro.
READ MORE CIPRO LEGAL NEWS
Cut to the summer of 2008. On July 8—two years after Public Citizen filed its petition and three years after the Office of the Attorney General for Illinois, the FDA finally ordered new warnings for Cipro and similar antibiotics in the fluoroquinolone class.
"We have seen continuing reports of tendon rupture so we are trying to increase awareness," said Dr. Edward Cox, director of the FDA's Office of Antimicrobial Products, in a statement released at the time.
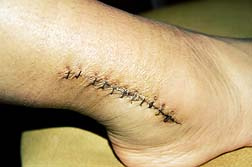
A year later, in the summer of 2009 patients are still reporting tendon rupture and other Cipro side effects inherent with the Cipro antibiotic—something that was known to be a risk 13 summers ago.
