LAWSUITS NEWS & LEGAL INFORMATION
Recalled St. Jude Medical Riata Defibrillator (ICD) Leads Legal News Articles & Interviews
Faulty St. Jude Riata Defibrillator Leads Eyed for Link to Heart Infections
 September 11, 2017. By Anne Wallace.
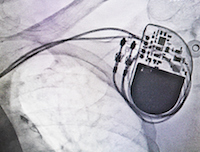
September 11, 2017. By Anne Wallace.Washington, DC: Premature Insulation Failure in recalled St. Jude Medical Riata Defibrillator (ICD) Leads has been tentatively linked to polymicrobial endocarditis, a serious, often deadly condition that causes inflammation of the heart lining, muscles and valves. The decayed insulation on the electrical leads that connect the pacemaker to a patient’s heart may be a fertile breeding ground for bacteria and fungi that can attack a patient’s heart.
Patients who still carry recalled St. Jude Defibrillators leads in their chests have many reasons to be wary. This is another one that needs to be watched. Left untreated, endocarditis is always fatal.
Patients suffering from the condition may experience:
- High fever;
- New or different heart murmur;
- Fatigue;
- Muscle pain;
- Small spots from broken blood vessels under the nails, on the whites of the eyes, on the chest, in the roof of the mouth and inside the cheeks;
- Chest pain, shortness of breath, coughing;
- Night sweats;
- Headache;
- Blood in the urine; or
- Unexpected weight loss.
In previous studies, endocarditis has been linked to intravenous drug abuse, dental infections and surgical procedures. Having had heart surgery, including a pacemaker implant has long been recognized as increasing a patient’s risk for the condition. The infectious agent in most of the occurrences is streptococcal or staphylococcal bacteria or fungi, but for the most part, infections may be traced to a single source.
The studies of endocarditis in patients with premature insulation failure in recalled St. Jude Medical Riata defibrillator (ICD) leads shows something different. Polymicrobial endocarditis arises from several infectious agents.
There is, for some reason, more variety in the flora (referred to in the scientific literature as “vegetations”) involved in these cases. The findings suggest that, somewhere in the patient’s body, there is a fertile place for bacteria and fungi to flourish. The inquiry has moved to the possibility that the faulty insulation and resulting exposure (or externalization) of St. Jude Medical Riata defibrillator leads may be the host.
The
Insulation Failure, Battery Failure and Cyber Hacking - A Triple Threat for Pacemaker Patients
 August 24, 2017. By Anne Wallace.
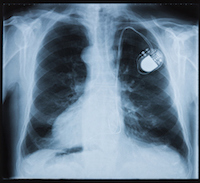
August 24, 2017. By Anne Wallace.Washington, DC: Premature insulation failure in recalled St. Jude Medical Riata defibrillator (ICD) leads may be only the tip of the iceberg for heart patients with ICDs, more commonly known as “pacemakers.”
In 2011 St. Jude Medical Inc. recalled the electrical leads used to connect the Riata pacemaker to patients’ hearts because of flaws in the protective insulation. Failure of the insulation on an electrical lead can cause a pacemaker to deliver inappropriate stimulation to the user’s heart. For cardiac patients with abnormal heart rhythms, the disruption can be fatal. In addition, the exposure of the electrical lead has been linked to polymicrobial endocarditis, a serious
The problems with St. Jude ICDs aren’t limited to the electrical leads. In 2016, the FDA issued a safety warning about the lithium batteries used to power them.
The ICDs are designed to alert patients to seek medical intervention to replace the battery approximately three months before it fails. St Jude has reported, however, that deposits of lithium, known as “lithium clusters,” can form within the battery, creating abnormal electrical connections that cause the battery to fail quickly. In some cases, full battery drainage has occurred in as a little as a day, leaving the patient with little or no time to get medical attention.
Finally, cybersecurity researchers at the University of Leuven in Belgium and the University of Birmingham in England have reported that they were able to successfully hack into ten different ICDs using relatively inexpensive, easily obtainable electronic components and a radio antenna.
In January 2017, the FDA warned that vulnerabilities in St. Jude Medical's ICDs might allow a hacker to deplete the battery or administer incorrect pacing or shocks. The hacker’s access point is a remote device programmer, intended to allow doctors and other medical professionals to adjust the settings on the pacemaker from up to five meters away.
St. Jude thereafter developed a patch to fix the devices’ susceptibility, but a later FDA warning letter suggests that the patch, itself, has not been adequately tested.
Patients who have an ICD manufactured by St. Jude Medical, Inc. would do well to seek medical advice about their risks and options.
Implantable Defibrillators with Faulty Batteries Lead to Class Action
 August 12, 2017. By Brenda Craig.
August 12, 2017. By Brenda Craig.Toronto, Ontario Thousands of Canadians and American cardiac arrhythmia patients with implantable defibrillators powered by faulty ion batteries and manufactured by St. Jude’s Medical Inc. and St. Jude’s Medical Canada are or will soon be eligible to join class actions suits in Canada and the US.
Read [ Implantable Defibrillators with Faulty Batteries Lead to Class Action ]
US Food and Drug Administration, FDA, St. Jude Medical Inc.
 February 12, 2017. By Gordon Gibb.
February 12, 2017. By Gordon Gibb.Washington, DC: The manufacturer of an implantable cardiac device recently caught in the crosshairs of a cybersecurity concern issued from the US Food and Drug Administration (FDA), continues to deal with reports of premature failure of ICD batteries. St. Jude Medical Inc. warned in early October that while the situation, in its estimation is rare, the early failure of batteries in their implanted defibrillators have caused two deaths.
Read [ US Food and Drug Administration, FDA, St. Jude Medical Inc. ]
