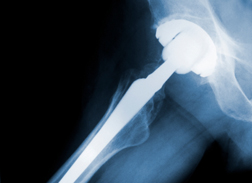
 The defective hip lawsuits centered upon the troubled Pinnacle line of newer-generation implants that DePuy removed from the market in 2013 - specifically, the Pinnacle Acetabular Cup implant system. The latter was a metal-on-metal device; the likes of which have proven problematic amidst a high failure rate and the potential for the implants to foster metallosis, a byproduct of wear involving cobalt and chromium alloys used in the manufacturing process. Such wear has succeeded, according to allegations made by plaintiffs, in the introduction of minute cobalt and chromium particles into surrounding tissue and ultimately into the bloodstream that results in metallosis toxicity.
The defective hip lawsuits centered upon the troubled Pinnacle line of newer-generation implants that DePuy removed from the market in 2013 - specifically, the Pinnacle Acetabular Cup implant system. The latter was a metal-on-metal device; the likes of which have proven problematic amidst a high failure rate and the potential for the implants to foster metallosis, a byproduct of wear involving cobalt and chromium alloys used in the manufacturing process. Such wear has succeeded, according to allegations made by plaintiffs, in the introduction of minute cobalt and chromium particles into surrounding tissue and ultimately into the bloodstream that results in metallosis toxicity.The latter can have serious health consequences. Together with inflamed tissue surrounding the failed implant, the pain and reduced mobility resulting from partial or total implant failure have combined to require revision surgery that is usually more complex, requires more healing time and is often quite soon after the initial implant procedure.
A defective hip implant lawsuit often results, as plaintiffs attempt to recover lost wages from additional time off work, along with compensation for pain and suffering. Hip and knee implants, historically, are designed to last upwards of 15 years under normal use.
The five plaintiffs in the Texas case are Margaret Aoki, Jay Christopher, Donald Greer, Richard Klusmann and Robert Peterson. They maintained in their action that DePuy failed to provide adequate warnings to consumers about a product alleged to be defective and largely untested, thanks in part to a 510(k) clearance for the product secured by the manufacturer.
READ MORE DEFECTIVE HIP IMPLANT LEGAL NEWS
Such products cleared through a 510(k) do not require clinical trials, and do not as a rule require pre-market approval.
The federal jury in Texas found that various circumstances suggested by the foregoing contributed to the pain and suffering alleged by the five plaintiffs in their defective hip implant litigation. The jury award, announced on March 17 of this year, amounted to $142 million in actual damages together with $360 million in punitive damages assessed against the defendants.
There are some 8,300 cases of similar import and merit presently centralized in US District Court, Northern District of Texas.
