 What is Bextra and why is it prescribed?
What is Bextra and why is it prescribed?Bextra (generic name Valdecoxib) is a non-steroidal anti-inflammatory prescription drug used to control tissue inflammation. It is in a group of drugs known as cox-2 inhibitors, prescribed to treat osteoarthritis, menstrual cramps, rheumatoid arthritis and adult pain management.
Traditionally, patients with these symptoms were given non-steroid anti-inflammatory drugs, but they affected both the cox-1 enzyme and the cox-2 enzyme produced by the body: this caused many patients to suffer chronic gastric problems. Because Bextra and other cox-2 inhibitors just affected the cox-2 enzyme, gastric side effects were minimal. Bextra impedes the production of certain chemical messengers responsible for pain and swelling by blocking the cox-2 enzyme.
When was Bextra linked to SJS?
Bextra side effects are much worse than stomach problems, which were caused by the drugs it replaced. Bextra is linked to Stevens Johnson Syndrome and TEN, Toxic Epidermal Necrolysis, allegedly even before FDA approval in November 2001. Bextra SJS lawsuits claim that both the FDA and Pfizer officials knew about Bextra's deadly side effects before the drug was marketed.
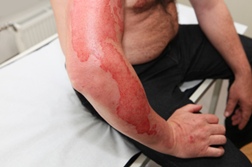
Many drugs cause SJS—a severe allergic reaction. Is Bextra worse than others?
According to the Stevens Johnson Syndrome Foundation, SJS is likely to occur more often for patients taking Bextra than for other cox-2 drugs and more often than traditional Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) such as Advil ibuprofen. As well, the most severe SJS cases have been linked to Bextra, along with NSAID Ibuprofen and Dilantin, another cox-2 inhibitor.
How dangerous is Bextra SJS?
SJS can be life-threatening: up to 27 percent of those diagnosed with the severe allergic reaction will incur long-term eye damage or vision loss, and up to 15 percent die.
Why is Bextra SJS considered a rare disease?
Increasingly, more people and health practitioners are aware of SJS symptoms, and they are becoming aware of the drugs, such as Bextra, that cause SJS. In the New England Journal of Medicine (March 11, 2010), an article by oncologist Dr. Ethan Basch put the problem with recognition of SJS succinctly: "Doctors, researchers, drug makers and regulators should pay more attention to patients' firsthand reports of their symptoms while they take medicines, because their information could help to guide treatment and research, and uncover safety problems."
Furthermore, he added that the medical community "systematically downgrade the severity of patients' symptoms'' and sometimes miss side effects altogether." One result is "preventable adverse events"—such as the early symptoms of SJS.
What has the FDA done about Bextra?
By November 2002, the FDA had received about 20 reports of serious reactions, including SJS and TEN, from Bextra. (About one month earlier, the European Medicines Evaluation Agency—the European counterpart to the FDA—issued a public statement warning of identical Bextra side effects.)
The FDA ordered Pfizer to add a black box warning to Bextra packaging, providing information and warning about the risks, including Stevens Johnson Syndrome and heart attack and strokes, but for many SJS victims, the warning was too late.
In April 2005, the agency asked Pfizer to pull Bextra from the market. Although Pfizer agreed to suspend sales and marketing, a company statement said it "respectfully disagrees" with the FDA's view of Bextra's risks, which included SJS. Pfizer also said it would discuss with the agency "ways to let the company restore Bextra's availability to doctors and patients."
Also in 2005, the FDA reported an "abnormally high number" of SJS and TEN incidents related to Bextra.
Currently, the FDA encourages doctors and patients to report problems that they think are adverse events from drugs already on the market to MedWatch. According to The International Herald Tribune (April 15, 2010), Dr. Ethan Basch said, "for example, in the postmarket setting [at Medwatch] we could ask 5,000 selected patients starting Bextra to report monthly (you would have reported the mouth sore [an early SJS symptom] without knowing if relevant or not, and this would then be pieced together with other reports).''
I heard that Pfizer settled some lawsuits—were they connected with Bextra?
READ MORE BEXTRA LEGAL NEWS
In October 2008, Pfizer agreed to $894 million to settle Bextra cardiovascular disease lawsuits. In September 2009, Pharmacia and Upjohn Company, a subsidiary of Pfizer, pled guilty for misbranding Bextra with the intent to defraud or mislead. The company agreed to pay criminal fines of $1.195 billion, the largest fine ever paid in the US. Additionally, Pfizer agreed to pay an additional one billion dollars plus interest to settle civil allegations that it fraudulently promoted and marketed Bextra and three other of its drugs. The company had promoted off-label uses of the drug since 2002.What does a Bextra lawsuit allege?
Bextra SJS attorneys allege that the manufacturer knew of serious health risks, yet failed to disclose such risks to the public. Among the serious diseases linked, none were effectively disclosed in the warnings. As well, Pfizer could be liable for any injury or death associated with using Bextra due to its inability or unwillingness to properly protect its consumers from the potentially fatal side effects of SJS and TEN. It is advisable that you contact an attorney to learn more about your rights and the possibility of filing a Bextra SJS claim.




