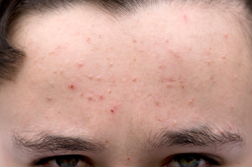
 The news source reported that Auckland-based Douglas Pharmaceuticals is looking to enter the market that accounts for 36 percent of the $856 billion worldwide pharmaceutical industry, as the company is hoping to sell its new acne drug after getting approval from the US Food and Drug Administration.
The news source reported that Auckland-based Douglas Pharmaceuticals is looking to enter the market that accounts for 36 percent of the $856 billion worldwide pharmaceutical industry, as the company is hoping to sell its new acne drug after getting approval from the US Food and Drug Administration.According to the news source, it took the company roughly 10 years to get approval from the FDA to sell its generic formulation of the acne drug isotretinoin in the US. Douglas recently announced that it would dispatch its first orders to America in March, after receiving the final thumbs-up from the organization.
Director Jeff Douglas said the company sold VersaPharm, a Marietta, Georgia-based firm, the exclusive rights to market its drug in the US, as the medication will be manufactured in Auckland. The American company has set its eyes on taking a 20 percent share of the $400 million acne medication market, as it will be competing with two other generic isotretinoin brands, according to the news source.
READ MORE ACCUTANE IBD LEGAL NEWS
Douglas noted that the grueling FDA approval process presented the company many challenges.
"We had to do new clinical trials on the product—there's a lot of extra information they [the FDA] require," he told the news source.
Isotretinoin was previously sold in the US under the name Accutane, and a number of lawsuits were filed due to the alleged side effects of the drug. According to The Louisiana Record, an Assumption Parish, Lousiana resident filed a lawsuit because he alleged it led to his diagnosis of Crohn's Disease.
