LAWSUITS NEWS & LEGAL INFORMATION
Stevens Johnson Syndrome Linked to Dilantin
The anti-epileptic drug Dilantin has been linked to Stevens Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN). More than 20 percent of TEN cases, which can develop from SJS, are believed to be caused by SJS Dilantin.
FREE DILANTIN SJS LAWSUIT EVALUATION
Send your Dilantin SJS claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Dilantin Stevens Johnson Syndrome
Dilantin is one of the most commonly prescribed anti-seizure medications in the US and has been marketed by Pfizer and its predecessor companies since 1936. A study that included 15 US burn centers showed that more than 20 percent of TEN cases are believed to be caused by Dilantin, with fatalities in approximately 1 of every 4 cases.
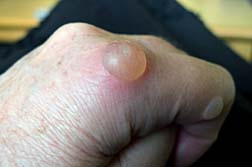 Along with SJS, other adverse drug reactions include Toxic Epidermal Necrolysis Symptoms (TEN) and Lyell's Syndrome. SJS is treated by hospitalization but most importantly, the cause must be determined first. If Dilantin is the cause, the medication must be discontinued immediately. If SJS is drug related, the chances of recovery are greatly increased once the drug is recognized. SJS can be fatal in about 15 percent of sufferers.
Along with SJS, other adverse drug reactions include Toxic Epidermal Necrolysis Symptoms (TEN) and Lyell's Syndrome. SJS is treated by hospitalization but most importantly, the cause must be determined first. If Dilantin is the cause, the medication must be discontinued immediately. If SJS is drug related, the chances of recovery are greatly increased once the drug is recognized. SJS can be fatal in about 15 percent of sufferers.
Allergic drug reactions are a major cause of death in the United States, causing more than 100,000 deaths among patients each year.
Stevens Johnson Syndrome can begin with a fever, sore throat and headache and over a few days or minutes can turn into skin lesions and blisters.
Stevens Johnson Syndrome is characterized by inflammation of the mucous membranes of the mouth, throat, eyes, genital tract and intestinal tract. Ulcers inside the mouth are the most common, irritation to the throat, tongue, gums, and lips. Affected individuals may also have skin lesions, blisters and bleeding in the lips, eyes, mouth, nasal passage and genital areas.
As Stevens Johnson Syndrome evolves, the skin literally sloughs off in sheets. Patients are typically treated in a hospital's burn unit. If the skin lesions become infected, or the patient develops lesions in the lungs, it can cause death.
Possible side effects of Dilantin can include:
- Liver Damage
- Rash
- Hallucinations
- Irregular Heartbeat
- Decreased Blood Pressure
- Increased Blood Sugar
- Birth Defects
Dilantin SJS Lawsuits
Following a hearing before a New York State Supreme Court Judge in October 2010, Pfizer will pay a $3.78 million settlement to the family.
Dilantin SJS and TEN Class Action Lawsuit
The lawsuit further alleges that the manufacturers knew of the drugs' dangers, but failed to adequately warn, and that Pfizer (in its submission on Bextra to Health Canada) said that drugs containing Dilantin substantially increased the risk of adverse skin reactions more so than other drugs on the market.
The plaintiffs are claiming strict products liability, negligence, failure to warn, negligence in bringing Dilantin to market, negligent misrepresentation, misrepresentation by omission, negligence per se, fraud and misrepresentation, fraud by concealment, violation of the Illinois Consumer Fraud Act and wrongful death.
Generic Dilantin SJS Lawsuit
Charles Henderson of Georgia took both generic Dilantin and generic fosphenytoin. Dilantin (phenytoin). The lawsuit notes that there have been more than 1,000 SJS cases from Dilantin and at least 139 deaths from SJS. Although the FDA acknowledged Dilantin' link to SJS and TENS in 2008, Henderson says there should have been more warning about the risk of getting SJS.
Dilantin SJS Legal Help
If you or a loved one has suffered from Stevens Johnson Syndrome after taking Dilantin, please click the link below to send your complaint to a lawyer to evaluate your claim at no cost or obligation.Last updated on
DILANTIN SJS LEGAL ARTICLES AND INTERVIEWS
Boy Narrowly Survives Stevens Johnson Syndrome Ordeal

Dilantin 'Dangerous and Lethal Drug:' Lawsuit Says

25 Percent of Dilantin TEN Patients Die, Study Finds

November 22, 2012
An eleven-year-old boy narrowly survived his ordeal with Stevens Johnson syndrome after taking ibuprofen, one of the drugs that has been linked to SJS and its more severe form, toxic epidermal necrolysis (TEN). Meanwhile, Dilantin, another drug that has been linked to SJS and TEN, is the focus of an SJS lawsuit alleging the drug is unreasonably dangerous. READ MORE
Dilantin 'Dangerous and Lethal Drug:' Lawsuit Says

July 26, 2012
Various pharmaceutical drugs can trigger Stevens Johnson Syndrome. Dilantin is among them. Thus, Dilantin SJS is an issue that should be of concern to any patient prescribed to take Dilantin, given the possibility of a life-changing or even life-threatening illness. READ MORE
25 Percent of Dilantin TEN Patients Die, Study Finds

April 25, 2012
Drugs are supposed to help, not hurt - at least that's what we are led to believe and have come to know. Little wonder that Americans continue to be blindsided by the tragic consequences that stem from adverse effects, such as that which is evidenced with Dilantin SJS. READ MORE
READ MORE Stevens Johnson Syndrome (SJS) Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Maryland
on
Texas
on
Arizona
on
North Carolina
on