LAWSUITS NEWS & LEGAL INFORMATION
Byetta Pancreatic Cancer, Byetta Thyroid Cancer
Read our Byetta FAQ
Were you looking for Actos or Diabetes Medication Lawsuits or Januvia or Victoza Side Effects lawsuits?
The use of Byetta has been linked to impaired kidney dysfunction and renal kidney failure as well as serious Byetta side effects including acute pancreatitis. Additionally, a recent UCLA study showed a possible increase in Byetta pancreatic cancer and Byetta thyroid cancer. Byetta attorneys are investigating lawsuits for users diagnosed with Impaired Kidney Function; Kidney Failure; Acute Pancreatitis; Hemorrhagic Pancreatitis; Necrotizing Pancreatitis and certain cancers. A Byetta lawyer can evaluate your potential Byetta lawsuit.
 Byetta (exenatide) is a type 2 diabetes drug that is injected twice a day to help reduce blood sugar levels in people with type 2 diabetes. It belongs to a class of drugs called incretin mimetics, which mimic the action of incretin hormones like GIP and GLP-1, which are found in the gastrointestinal tract.
Byetta (exenatide) is a type 2 diabetes drug that is injected twice a day to help reduce blood sugar levels in people with type 2 diabetes. It belongs to a class of drugs called incretin mimetics, which mimic the action of incretin hormones like GIP and GLP-1, which are found in the gastrointestinal tract.
Byetta was approved by the FDA in April 2005. Since that time the FDA received at least 78 reports of kidney problems related to Byetta, including kidney failure. Although some patients already had kidney disease before they started Byetta, others developed kidney problems after taking the drug. Overall, 91 percent of patients of the 78 reported were hospitalized, 18 required dialysis, two had kidney transplants, and four died. Almost half of those patients who stopped taking Byetta eventually had improved kidney function.
Potential symptoms of Byetta kidney problems could include: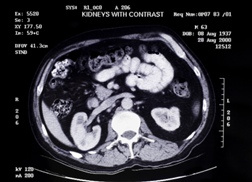 In November 2009 the FDA announced that new prescribing information would be added about reports of Byetta side effects linked to kidney failure and other kidney problems after receiving at least 78 reports from users experiencing altered kidney function, including at least 62 reports of renal kidney failure on Byetta. Patients who have been prescribed Byetta should notify their doctor upon experiencing any of the above Byetta side effects that would indicate possible renal failure or kidney problems.
In November 2009 the FDA announced that new prescribing information would be added about reports of Byetta side effects linked to kidney failure and other kidney problems after receiving at least 78 reports from users experiencing altered kidney function, including at least 62 reports of renal kidney failure on Byetta. Patients who have been prescribed Byetta should notify their doctor upon experiencing any of the above Byetta side effects that would indicate possible renal failure or kidney problems.
A recent study published in the journal, Gastroenterology raised some red flags concerning the safety of the diabetes drug exenatide (Byetta), as well as for diabetes drug sitagliptin (Januvia.)
The study was conducted by Peter Butler, MD of the University of California Los Angeles (UCLA), and colleagues and showed a six-fold increased risk for pancreatitis with either drug when compared with controls, as well as a three-fold greater chance of pancreatic cancer. The UCLA exenatide and sitagliptin study also found a four-fold greater likelihood of reported thyroid cancer with exenatide.
Dr. Butler and his colleagues evaluated data from the FDA AERS (Adverse Event Reporting System) between 2004 and 2009, which are limited, but may indicate cause for concern.
 In October 2007 the FDA also noted 30 reports of acute pancreatitis, which is sudden inflammation of the pancreas, in patients taking Byetta. (None of those patients had hemorrhagic or necrotizing pancreatitis.) The manufacturer agreed to update the warning label about this side effect.
In October 2007 the FDA also noted 30 reports of acute pancreatitis, which is sudden inflammation of the pancreas, in patients taking Byetta. (None of those patients had hemorrhagic or necrotizing pancreatitis.) The manufacturer agreed to update the warning label about this side effect.
In August 2008 the FDA sent out a second alert to healthcare providers, informing them that six additional cases of necrotizing pancreatitis or hemorrhagic pancreatitis had been reported among Byetta users, two of which resulted in death. (Hemorrhagic pancreatitis is a severe form of the condition where massive erosion of blood vessels can lead to severe bleeding; Necrotizing pancreatitis involves tissue damage, which leads to the release of toxins and enzymes into the blood stream and may cause multi-organ failure and death.)
At that time, the FDA asked the manufacturers of Byetta to include information on acute pancreatitis in the "precautions" section of Byetta's label. The new label also included more information regarding patients with renal impairment.
Since 2005, the FDA has received 40 reports of Byetta patients developing acute pancreatitis after taking Byetta including 6 deaths. Of these, 21 diabetics required hospitalization. As of November 2009, at least 78 patients had reported kidney problems with Byetta use, including 62 cases of Byetta renal kidney failure. The FDA has responded to these adverse event reports with the following Byetta warnings:
October 16, 2007: after reviewing 30 cases of pancreatitis, the FDA mandated that Amylin and Eli Lilly include stricter, more prominent warning labels about possible Byetta side effects on the products' packaging including reports of acute pancreatitis.
August 18, 2008: the FDA issued an alert to healthcare providers about six additional cases of necrotizing or hemorrhagic pancreatitis among users of Byetta, two of which have resulted in death. The warning follows a prior alert issued by the FDA in October 2007, involving at least 30 other cases that suggested an association between the diabetes drug Byetta and pancreatitis.
November 2, 2009: the FDA acted on new safety information about possible kidney function problems, including renal kidney failure, in patients taking Byetta (exenatide) to treat Type 2 diabetes. From April 2005 through October 2008, the FDA received 78 reports of problems with kidney function in patients using Byetta. Some cases occurred in patients with pre-existing kidney disease or in patients with one or more risk factors for developing kidney problems.
 A number of attorneys say that Amylin Pharmaceuticals and Eli Lilly & Co. have failed to adequately warn about the risk of these serious and potentially fatal side effects.
A number of attorneys say that Amylin Pharmaceuticals and Eli Lilly & Co. have failed to adequately warn about the risk of these serious and potentially fatal side effects.
In October, just one month earlier, Amylin Pharmaceuticals, Inc. and Eli Lilly & Co., the manufacturers, announced that Byetta was approved for use as a stand-alone medication (monotherapy) along with diet and exercise to improve glycemic control in adults with type 2 diabetes. Previously, it was approved for use only in patients who were also taking other common diabetes medications and had not achieved adequate glycemic control. In January 2010, however, the FDA sent warnings to Amylin, Lilly, Bayer Healthcare Pharmaceuticals and Cephalon. In the letter to Amylin, the FDA cited the company for promoting Byetta as a stand-alone diabetes drug at the Endocrine Society's Annual Meeting, before it was approved as such.
According to Bloomberg, there was more trouble for Amylin. US regulators inspected the Amylin plant in March 2010 and reported it had inadequate quality control and training for employees.
In April 2010, Federal drug safety reviewers said that Byetta side effects may include thyroid cancer concerns of similar medications, which may result in black-box warning for a new long-acting version of Bydureon (also known as Byetta LAR), which has yet to be approved.
Diabetes affects more than 24 million people in the United States and an estimated 246 million adults worldwide. Approximately 90-95 percent of those affected have type 2 diabetes. Doctors wrote almost 7 million Byetta prescriptions between 2005 and 2008 and Byetta had worldwide sales of $796.5 million last year. Lilly markets the drug outside the US and co-markets it in the US with Amylin.
Last updated on
FREE BYETTA LAWSUIT EVALUATION
Send your Byetta claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Byetta Renal Kidney Failure
 Byetta (exenatide) is a type 2 diabetes drug that is injected twice a day to help reduce blood sugar levels in people with type 2 diabetes. It belongs to a class of drugs called incretin mimetics, which mimic the action of incretin hormones like GIP and GLP-1, which are found in the gastrointestinal tract.
Byetta (exenatide) is a type 2 diabetes drug that is injected twice a day to help reduce blood sugar levels in people with type 2 diabetes. It belongs to a class of drugs called incretin mimetics, which mimic the action of incretin hormones like GIP and GLP-1, which are found in the gastrointestinal tract.Byetta was approved by the FDA in April 2005. Since that time the FDA received at least 78 reports of kidney problems related to Byetta, including kidney failure. Although some patients already had kidney disease before they started Byetta, others developed kidney problems after taking the drug. Overall, 91 percent of patients of the 78 reported were hospitalized, 18 required dialysis, two had kidney transplants, and four died. Almost half of those patients who stopped taking Byetta eventually had improved kidney function.
Potential symptoms of Byetta kidney problems could include:
- Nausea, Vomiting and Diarrhea
- Changes in urine color and frequency of urination
- Fatigue
- Swelling of hands or feet
- Changes in appetite or digestion
- Dull aches in the mid to lower back
 In November 2009 the FDA announced that new prescribing information would be added about reports of Byetta side effects linked to kidney failure and other kidney problems after receiving at least 78 reports from users experiencing altered kidney function, including at least 62 reports of renal kidney failure on Byetta. Patients who have been prescribed Byetta should notify their doctor upon experiencing any of the above Byetta side effects that would indicate possible renal failure or kidney problems.
In November 2009 the FDA announced that new prescribing information would be added about reports of Byetta side effects linked to kidney failure and other kidney problems after receiving at least 78 reports from users experiencing altered kidney function, including at least 62 reports of renal kidney failure on Byetta. Patients who have been prescribed Byetta should notify their doctor upon experiencing any of the above Byetta side effects that would indicate possible renal failure or kidney problems.Study: Byetta Thyroid Cancer, Pancreatic Cancer
The study was conducted by Peter Butler, MD of the University of California Los Angeles (UCLA), and colleagues and showed a six-fold increased risk for pancreatitis with either drug when compared with controls, as well as a three-fold greater chance of pancreatic cancer. The UCLA exenatide and sitagliptin study also found a four-fold greater likelihood of reported thyroid cancer with exenatide.
Dr. Butler and his colleagues evaluated data from the FDA AERS (Adverse Event Reporting System) between 2004 and 2009, which are limited, but may indicate cause for concern.
Byetta Pancreatic Cancer
 In October 2007 the FDA also noted 30 reports of acute pancreatitis, which is sudden inflammation of the pancreas, in patients taking Byetta. (None of those patients had hemorrhagic or necrotizing pancreatitis.) The manufacturer agreed to update the warning label about this side effect.
In October 2007 the FDA also noted 30 reports of acute pancreatitis, which is sudden inflammation of the pancreas, in patients taking Byetta. (None of those patients had hemorrhagic or necrotizing pancreatitis.) The manufacturer agreed to update the warning label about this side effect.In August 2008 the FDA sent out a second alert to healthcare providers, informing them that six additional cases of necrotizing pancreatitis or hemorrhagic pancreatitis had been reported among Byetta users, two of which resulted in death. (Hemorrhagic pancreatitis is a severe form of the condition where massive erosion of blood vessels can lead to severe bleeding; Necrotizing pancreatitis involves tissue damage, which leads to the release of toxins and enzymes into the blood stream and may cause multi-organ failure and death.)
At that time, the FDA asked the manufacturers of Byetta to include information on acute pancreatitis in the "precautions" section of Byetta's label. The new label also included more information regarding patients with renal impairment.
Byetta FDA Warnings
October 16, 2007: after reviewing 30 cases of pancreatitis, the FDA mandated that Amylin and Eli Lilly include stricter, more prominent warning labels about possible Byetta side effects on the products' packaging including reports of acute pancreatitis.
August 18, 2008: the FDA issued an alert to healthcare providers about six additional cases of necrotizing or hemorrhagic pancreatitis among users of Byetta, two of which have resulted in death. The warning follows a prior alert issued by the FDA in October 2007, involving at least 30 other cases that suggested an association between the diabetes drug Byetta and pancreatitis.
November 2, 2009: the FDA acted on new safety information about possible kidney function problems, including renal kidney failure, in patients taking Byetta (exenatide) to treat Type 2 diabetes. From April 2005 through October 2008, the FDA received 78 reports of problems with kidney function in patients using Byetta. Some cases occurred in patients with pre-existing kidney disease or in patients with one or more risk factors for developing kidney problems.
Byetta Lawsuits
 A number of attorneys say that Amylin Pharmaceuticals and Eli Lilly & Co. have failed to adequately warn about the risk of these serious and potentially fatal side effects.
A number of attorneys say that Amylin Pharmaceuticals and Eli Lilly & Co. have failed to adequately warn about the risk of these serious and potentially fatal side effects.In October, just one month earlier, Amylin Pharmaceuticals, Inc. and Eli Lilly & Co., the manufacturers, announced that Byetta was approved for use as a stand-alone medication (monotherapy) along with diet and exercise to improve glycemic control in adults with type 2 diabetes. Previously, it was approved for use only in patients who were also taking other common diabetes medications and had not achieved adequate glycemic control. In January 2010, however, the FDA sent warnings to Amylin, Lilly, Bayer Healthcare Pharmaceuticals and Cephalon. In the letter to Amylin, the FDA cited the company for promoting Byetta as a stand-alone diabetes drug at the Endocrine Society's Annual Meeting, before it was approved as such.
According to Bloomberg, there was more trouble for Amylin. US regulators inspected the Amylin plant in March 2010 and reported it had inadequate quality control and training for employees.
In April 2010, Federal drug safety reviewers said that Byetta side effects may include thyroid cancer concerns of similar medications, which may result in black-box warning for a new long-acting version of Bydureon (also known as Byetta LAR), which has yet to be approved.
Diabetes affects more than 24 million people in the United States and an estimated 246 million adults worldwide. Approximately 90-95 percent of those affected have type 2 diabetes. Doctors wrote almost 7 million Byetta prescriptions between 2005 and 2008 and Byetta had worldwide sales of $796.5 million last year. Lilly markets the drug outside the US and co-markets it in the US with Amylin.
Byetta Legal Help
If you or a loved one has suffered from kidney problems, renal kidney failure or acute pancreatitis after taking Byetta, please click the link below to send your complaint to a lawyer to evaluate your claim at no cost or obligation.Last updated on
BYETTA LEGAL ARTICLES AND INTERVIEWS
Big Trial Coming for Diabetes Drug Byetta

Doctor Knew of Problems with Januvia in 2008

Januvia and Byetta Increase Pancreatic and Neuroendocrine Cancer Risk

September 23, 2013
More than seven million prescriptions have been written in the US for a diabetes drug called Byetta, and this coming November, all eyes will focus on a California court case that alleges that its use triggered acute pancreatitis in five individuals and led to the death of two others. READ MORE
Doctor Knew of Problems with Januvia in 2008

June 5, 2013
Washington, DC: Merck, the maker of sitagliptin (Januvia, Janumet, Janumet XR, Juvisync), is, according to The New York Times, facing 43 personal injury lawsuits alleging Januvia caused pancreatic cancer. But Merck is not the only pharmaceutical company facing legal action over adverse event profiles associated with this class of drugs: over 100 lawsuits claiming injury from Byetta, mostly pancreatitis, according to the latest quarterly regulatory filing from Bristol-Myers. READ MORE
Januvia and Byetta Increase Pancreatic and Neuroendocrine Cancer Risk

April 1, 2013
Results from a small study of organs from diabetic patients who were treated with Merck’s Januvia (sitagliptin) and Bristol-Myers Squibb’s Byetta and Bydureon (exenatide) show increases in pancreatic mass and beta cell mass, potentially increasing their risk for pancreatic cancer. READ MORE
READ MORE Diabetes Drugs Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Texas
on
Missouri
on
Georgia
on