LAWSUITS NEWS & LEGAL INFORMATION
Botox and Myobloc Linked to Paralysis and Death
On August 3, 2009 the FDA updated its safety alert to a Black Box Warning on the popular anti-wrinkle drugs Botox and Myobloc. All Botox product labels now warn that the effects of the botulinum toxin may spread from the area of injection to other areas of the body, causing symptoms similar to those of botulism. Those symptoms, which have mostly been seen in children with cerebral palsy who received injections off-label for treatment of muscle spasticity, include potentially life-threatening swallowing and breathing difficulties and even death.
FREE BOTOX AND MYOBLOC LAWSUIT EVALUATION
Send your Botox and Myobloc claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Black Box warnings have been added on four botulinum toxin drug products:
 Between Nov. 1, 1997, and Dec. 31, 2006, the advocacy group Public Citizen reported that 658 people had adverse reactions to botulinum toxin. Of these, 180 people had difficulty swallowing or contracted aspiration pneumonia when food refluxed into the windpipe. Eighty-seven of those people had to be hospitalized and 16 died. All but one of the deaths were associated with therapeutic use.
Between Nov. 1, 1997, and Dec. 31, 2006, the advocacy group Public Citizen reported that 658 people had adverse reactions to botulinum toxin. Of these, 180 people had difficulty swallowing or contracted aspiration pneumonia when food refluxed into the windpipe. Eighty-seven of those people had to be hospitalized and 16 died. All but one of the deaths were associated with therapeutic use.
The FDA was petitioned by Public Citizen to order a black box warning on all Botox products. (A black box warning is the FDA's strongest type: it appears on the package insert for prescription drugs that may cause serious adverse effects.) Public Citizen also requested that every patient receive a pamphlet outlining the risk before each botulinum toxin is administered.
The FDA issued a press release two weeks after the Public Citizen report, acknowledging safety concerns with the botulinum toxin products and revealing that the agency was conducting ongoing reviews of data from the drug companies as well as monitoring reports of adverse events related to the use of the products.
Botox Cerebral Palsy
Several deaths in children, mostly cerebral palsy patients who were administered Botox injections off-label for associated limb spasticity (severe arm and leg muscle spasms), have been reported. The use of these drugs off-label has not been approved by FDA. Although symptoms have also been reported in adults treated both for approved and unapproved uses, Cerebral palsy Botox treatments use much larger doses than cosmetic use of Botox and are applied to areas more likely to allow the toxin to spread to other parts of the body and thereby cause Botox problems.
Symptoms of Botox cerebral palsy treatment problems could include the following:
Botox and Myobloc use botulinum toxin, which blocks nerve impulses to muscles. The FDA only approved the use of botulinum toxin for a limited number of "therapeutic" conditions, including uncontrollable neck and shoulder muscle contractions, crossed eyes, spasmodic blinking of the eyes and excessive underarm sweating. The only approved cosmetic use is for temporary (up to 120 days) smoothing of wrinkles or frown lines between the eyebrows at the recommended doses. Most cosmetic uses of botulinum toxin are unapproved.
Use of botulinum toxins for treatment of limb spasticity in children or adults is not approved in the US, nor have botulinum toxins been approved in any condition in children less than 12 years of age.Myobloc, made by Solstice Neurosciences Inc., was approved in December 2000 for the treatment of adults with cervical dystonia, a movement disorder that causes the muscles to contract and spasm involuntarily.
Botox, manufactured by Allergan, was first approved in December 1989 to treat blepharospasm (spasm of the eyelids) and strabismus (misaligned eyes or "cross-eyed"). In 2000, Botox was approved to treat cervical dystonia (severe neck muscle spasms). In July 20, 2004 it was approved to treat severe primary axillary hyperhydrosis (excess sweating).
Botox and Myobloc still Available
Although Botox products now include a black box warning, healthcare officials have not advised discontinuing prescribing these products. However, the FDA has made the following recommendations to clinicians who administer Botox and/or Myobloc:
The Botox label currently warns of the potential for botulinum toxin to spread beyond the injection site and that systemic adverse effects, including severe difficulty swallowing and difficulty breathing and even death, have occurred in patients with neuromuscular disorders such as myasthenia gravis.
 The FDA is also warning the public that the botulism toxin seems to be harming people who don't necessarily have neuromuscular diseases. The agency has been investigating reports of illnesses in adults who used the drugs for other conditions, including a woman who was hospitalized after she was given Botox for forehead wrinkles.
The FDA is also warning the public that the botulism toxin seems to be harming people who don't necessarily have neuromuscular diseases. The agency has been investigating reports of illnesses in adults who used the drugs for other conditions, including a woman who was hospitalized after she was given Botox for forehead wrinkles.
The FDA has asked Allergan Inc. and Solstice Neurosciences Inc., makers of the Botox and Myobloc injectables, to provide additional safety records. A Solstice spokesperson said that children with cerebral palsy receive far larger doses injected into their leg muscles than the doses given adults for cosmetic purposes. The FDA said the problems may be related to overdoses but it also has reports of side effects with a variety of doses.
If you have received a botulinum toxin injection for any reason—cosmetic or medical—you should seek immediate care if you have suffered any symptoms of botulism, including difficulty swallowing or breathing; slurred speech; muscle weakness or difficulty holding up your head. And you may also wish to seek legal help.
- Botox (new established name: onabotulinumtoxinA)
- Botox Cosmetic (new established name: onabotulinumtoxinA)
- Myobloc (new established name: rimabotulinumtoxinB)
- Dysport (abobotulinumtoxinA) was approved in April 2009 with the boxed warning and is not making any name or label changes at this time.
 Between Nov. 1, 1997, and Dec. 31, 2006, the advocacy group Public Citizen reported that 658 people had adverse reactions to botulinum toxin. Of these, 180 people had difficulty swallowing or contracted aspiration pneumonia when food refluxed into the windpipe. Eighty-seven of those people had to be hospitalized and 16 died. All but one of the deaths were associated with therapeutic use.
Between Nov. 1, 1997, and Dec. 31, 2006, the advocacy group Public Citizen reported that 658 people had adverse reactions to botulinum toxin. Of these, 180 people had difficulty swallowing or contracted aspiration pneumonia when food refluxed into the windpipe. Eighty-seven of those people had to be hospitalized and 16 died. All but one of the deaths were associated with therapeutic use.
The FDA was petitioned by Public Citizen to order a black box warning on all Botox products. (A black box warning is the FDA's strongest type: it appears on the package insert for prescription drugs that may cause serious adverse effects.) Public Citizen also requested that every patient receive a pamphlet outlining the risk before each botulinum toxin is administered.
The FDA issued a press release two weeks after the Public Citizen report, acknowledging safety concerns with the botulinum toxin products and revealing that the agency was conducting ongoing reviews of data from the drug companies as well as monitoring reports of adverse events related to the use of the products.
Botox Cerebral Palsy
Several deaths in children, mostly cerebral palsy patients who were administered Botox injections off-label for associated limb spasticity (severe arm and leg muscle spasms), have been reported. The use of these drugs off-label has not been approved by FDA. Although symptoms have also been reported in adults treated both for approved and unapproved uses, Cerebral palsy Botox treatments use much larger doses than cosmetic use of Botox and are applied to areas more likely to allow the toxin to spread to other parts of the body and thereby cause Botox problems.
Symptoms of Botox cerebral palsy treatment problems could include the following:
- Weakness
- Double or Blurred Vision
- Drooping Eyelids
- Slurred Speech
- Dry Mouth
- Difficulty Swallowing
- Respiratory Distress
Botox and Myobloc use botulinum toxin, which blocks nerve impulses to muscles. The FDA only approved the use of botulinum toxin for a limited number of "therapeutic" conditions, including uncontrollable neck and shoulder muscle contractions, crossed eyes, spasmodic blinking of the eyes and excessive underarm sweating. The only approved cosmetic use is for temporary (up to 120 days) smoothing of wrinkles or frown lines between the eyebrows at the recommended doses. Most cosmetic uses of botulinum toxin are unapproved.
Use of botulinum toxins for treatment of limb spasticity in children or adults is not approved in the US, nor have botulinum toxins been approved in any condition in children less than 12 years of age.Myobloc, made by Solstice Neurosciences Inc., was approved in December 2000 for the treatment of adults with cervical dystonia, a movement disorder that causes the muscles to contract and spasm involuntarily.
Botox, manufactured by Allergan, was first approved in December 1989 to treat blepharospasm (spasm of the eyelids) and strabismus (misaligned eyes or "cross-eyed"). In 2000, Botox was approved to treat cervical dystonia (severe neck muscle spasms). In July 20, 2004 it was approved to treat severe primary axillary hyperhydrosis (excess sweating).
Botox and Myobloc still Available
Although Botox products now include a black box warning, healthcare officials have not advised discontinuing prescribing these products. However, the FDA has made the following recommendations to clinicians who administer Botox and/or Myobloc:
- Realize that potency determinations (units) vary among the botulinum toxin products and that clinical doses expressed in units are not comparable from one botulinum product to the next.
- Remain vigilant for systemic effects that may follow administration of botulinum toxins, including dysphagia, dysphonia, weakness, dyspnea, or respiratory distress. These symptoms, which are suggestive of botulism, have been reported from 1 day to several weeks after treatment.
- Inform patients and caregivers regarding how to recognize the signs and symptoms of systemic effects after being injected with a botulinum toxin. Advise patients to seek immediate medical attention for worsening or unexpected difficulty swallowing or talking, trouble breathing, or muscle weakness.
The Botox label currently warns of the potential for botulinum toxin to spread beyond the injection site and that systemic adverse effects, including severe difficulty swallowing and difficulty breathing and even death, have occurred in patients with neuromuscular disorders such as myasthenia gravis.
 The FDA is also warning the public that the botulism toxin seems to be harming people who don't necessarily have neuromuscular diseases. The agency has been investigating reports of illnesses in adults who used the drugs for other conditions, including a woman who was hospitalized after she was given Botox for forehead wrinkles.
The FDA is also warning the public that the botulism toxin seems to be harming people who don't necessarily have neuromuscular diseases. The agency has been investigating reports of illnesses in adults who used the drugs for other conditions, including a woman who was hospitalized after she was given Botox for forehead wrinkles.
The FDA has asked Allergan Inc. and Solstice Neurosciences Inc., makers of the Botox and Myobloc injectables, to provide additional safety records. A Solstice spokesperson said that children with cerebral palsy receive far larger doses injected into their leg muscles than the doses given adults for cosmetic purposes. The FDA said the problems may be related to overdoses but it also has reports of side effects with a variety of doses.
If you have received a botulinum toxin injection for any reason—cosmetic or medical—you should seek immediate care if you have suffered any symptoms of botulism, including difficulty swallowing or breathing; slurred speech; muscle weakness or difficulty holding up your head. And you may also wish to seek legal help.
Botox and Myobloc Legal Help
If you or a loved one has suffered paralysis or death as a result of Botox or Myobloc, please click the link below to send your complaint to a lawyer to evaluate your claim at no cost or obligation.Last updated on
BOTOX AND MYOBLOC LEGAL ARTICLES AND INTERVIEWS
Botox Approved for Migraine
Attorney Ray Chester Is the Botox Buster
Indiana Couple Blames Botox for Death of Daughter

October 16, 2010
On the heels Allergan Inc. pleading guilty to marketing its wrinkle smoother botox for unapproved uses, the Food and Drug Administration has now approved Allergan's product, which is a purified form of the poison botulinum, for chronic migraine headaches. READ MORE
Attorney Ray Chester Is the Botox Buster

May 27, 2010
Attorney Ray Chester has given Allergan, the manufacturer of Botox, a few worry lines of its own. He recently obtained a $15 million verdict on behalf of an Oklahoma woman who suffered unintended consequences after receiving a Botox injection to eliminate wrinkles in her forehead. Chester has at least eight other suits pending against the company. READ MORE
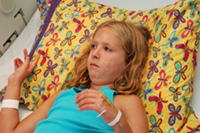
Indiana Couple Blames Botox for Death of Daughter

March 20, 2010
The US Food and Drug Administration (FDA) has approved Botox injections for only a handful of indications, but off-label use continues on a wide scale. According to a proposed lawsuit in Indiana, off-label use of Botox for muscle spasms triggered the death of a 10-year-old girl with cerebral palsy. READ MORE
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Angela johnson
on
Gloria Hill
on
Dawn
on
New Jersey
on
Illinois
on
Missouri
on
North Carolina
on
California
on
California
on
Alabama
on