LAWSUITS NEWS & LEGAL INFORMATION
Accutane Acne Medication and Severe Side Effects
Accutane acne medication is reported to have severe side effects including increased risk of inflammatory bowel disease (IBD), birth defects, depression and suicide. Recently, Health Canada warned about a risk of serious skin reactions. These Accutane side effects have resulted in lawsuits being filed against the manufacturers of the Accutane medication. Although Accutane has been pulled from the market, at the manufacturer's request, lawsuits are still pending.
Accutane was prescribed for the treatment of severe acne or recalcitrant cystic nodular acne. It was often prescribed as the next-level treatment for acne after a course of Tetracycline had failed to diminish acne breakouts. Accutane worked by shrinking the skin's oil glands and diminishing their oil output. Accutane was a capsule taken orally every day for several months. Accutane, generically known as isotretinoin, was a synthetic derivative of vitamin A.
 Accutane was first manufactured and distributed by Hoffman-LaRoche in 1982. Before it was pulled from the market, Accutane reportedly had annual sales of more than $1.2 billion. Accutane was pulled from the market by its manufacturer, Hoffman-LaRoche, who cited market pressure as the reason for pulling the medication.
Accutane was first manufactured and distributed by Hoffman-LaRoche in 1982. Before it was pulled from the market, Accutane reportedly had annual sales of more than $1.2 billion. Accutane was pulled from the market by its manufacturer, Hoffman-LaRoche, who cited market pressure as the reason for pulling the medication.
With reports of medical problems and side effects linked to Accutane, the FDA was involved in alerting the public and promoting changes to the warning label. The FDA has put limitations on prescribing and filling Accutane prescriptions to avoid abuse and unauthorized use of the potentially dangerous drug.
Although Accutane has been pulled from the market, generic versions of isotretinoin, including Amnesteem, Claravis and Sotret, are still available on the market.
Accutane's side effects include inflammatory bowel disease, risk of birth defects, increased risk of suicide and a risk of serious skin reactions.
Accutane and Inflammatory Bowel Disease (IBD)
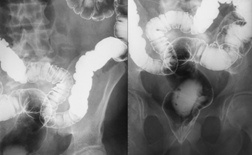 Accutane was linked to an inflammatory condition that occurs in the patient's colon and small intestine. Patients who develop inflammatory bowel disease experience chronically red and swollen intestines, weight loss, bleeding and pain and cramping. Symptoms of inflammatory bowel disease generally appear within four years of the last exposure to Accutane. Treatment of inflammatory bowel disease can involve surgery to remove part or all of the patient's colon.
Accutane was linked to an inflammatory condition that occurs in the patient's colon and small intestine. Patients who develop inflammatory bowel disease experience chronically red and swollen intestines, weight loss, bleeding and pain and cramping. Symptoms of inflammatory bowel disease generally appear within four years of the last exposure to Accutane. Treatment of inflammatory bowel disease can involve surgery to remove part or all of the patient's colon.
Inflammatory bowel disease includes two different diseases: Crohn's disease and ulcerative colitis. Both affect the digestive system, although ulcerative colitis affects only the lining of the bowel, whereas inflammation from Crohn's disease can affect deep layers of the intestinal wall.
Patients with inflammatory bowel disease can develop serious problems including dehydration and anemia if the problem becomes severe. Problems associated with inflammatory bowel disease—such as diarrhea—can make it difficult for patients to continue on with their normal day-to-day living. Lawsuits have been filed against the maker of Accutane, alleging patients were not adequately warned about the risk of inflammatory bowel disease.
Accutane and Birth Defects
Shortly after international distribution, Accutane was in the news for allegations of causing severe birth defects when the mother took Accutane while pregnant. Birth defects included facial and bodily abnormalities and disorders of the brain, central nervous system, or cardiovascular system in the newborn baby.
In 2006, the FDA implemented the iPLEDGE program, designed to prevent the use of isotretinoin while pregnant. Patients who wanted to take Accutane were required to register for iPLEDGE, complete an informed consent form and, for women who were of childbearing age, comply with necessary pregnancy testing and be on two methods of birth control, unless the patient agreed to be abstinent.
Accutane and Suicide
 Before being removed from the market, Accutane was linked to an increased risk of depression, psychosis, suicidal ideation, suicide attempts and suicide. According to the FDA, from the time isotretinoin was marketed in 1982 through August 2004, there were 4,992 spontaneous reports of psychiatric disturbances associated with the use of isotretinoin in the US. Between 1982 and 2002 there were 165 reported suicides. However, the FDA noted that suicides linked to isotretinoin could be underreported, meaning there could be more than the 165 reported suicides. Prior to being removed from the market, Accutane the FDA warned that patients treated with isotretinoin should be observed for symptoms of depression or for suicidal thoughts.
Before being removed from the market, Accutane was linked to an increased risk of depression, psychosis, suicidal ideation, suicide attempts and suicide. According to the FDA, from the time isotretinoin was marketed in 1982 through August 2004, there were 4,992 spontaneous reports of psychiatric disturbances associated with the use of isotretinoin in the US. Between 1982 and 2002 there were 165 reported suicides. However, the FDA noted that suicides linked to isotretinoin could be underreported, meaning there could be more than the 165 reported suicides. Prior to being removed from the market, Accutane the FDA warned that patients treated with isotretinoin should be observed for symptoms of depression or for suicidal thoughts.
Accutane and Skin Reactions
In February, 2010, Health Canada and Hoffman-LaRoche issued a warning about the increased risk of serious skin reactions in patients who used Accutane. Those risks include Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Both conditions are rare but can be serious, resulting in hospitalization, disability or death.
Hoffmann-LaRoche is the U.S. subsidiary of "The Roche Group", a leading international health care company focused on pharmaceuticals, diagnostics and vitamins. The Roche Group has been in international news for fraudulent conduct, discovery abuses, and patent infringement, including fraud against the United States Trademark and Patent Office. In 1999, Roche budgeted over one billion dollars for criminal fines, penalties, and settlement of cases.
Last updated on
FREE ACCUTANE LAWSUIT EVALUATION
Send your Accutane claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Accutane
 Accutane was first manufactured and distributed by Hoffman-LaRoche in 1982. Before it was pulled from the market, Accutane reportedly had annual sales of more than $1.2 billion. Accutane was pulled from the market by its manufacturer, Hoffman-LaRoche, who cited market pressure as the reason for pulling the medication.
Accutane was first manufactured and distributed by Hoffman-LaRoche in 1982. Before it was pulled from the market, Accutane reportedly had annual sales of more than $1.2 billion. Accutane was pulled from the market by its manufacturer, Hoffman-LaRoche, who cited market pressure as the reason for pulling the medication.
With reports of medical problems and side effects linked to Accutane, the FDA was involved in alerting the public and promoting changes to the warning label. The FDA has put limitations on prescribing and filling Accutane prescriptions to avoid abuse and unauthorized use of the potentially dangerous drug.
Although Accutane has been pulled from the market, generic versions of isotretinoin, including Amnesteem, Claravis and Sotret, are still available on the market.
Accutane Side Effects
Accutane and Inflammatory Bowel Disease (IBD)
 Accutane was linked to an inflammatory condition that occurs in the patient's colon and small intestine. Patients who develop inflammatory bowel disease experience chronically red and swollen intestines, weight loss, bleeding and pain and cramping. Symptoms of inflammatory bowel disease generally appear within four years of the last exposure to Accutane. Treatment of inflammatory bowel disease can involve surgery to remove part or all of the patient's colon.
Accutane was linked to an inflammatory condition that occurs in the patient's colon and small intestine. Patients who develop inflammatory bowel disease experience chronically red and swollen intestines, weight loss, bleeding and pain and cramping. Symptoms of inflammatory bowel disease generally appear within four years of the last exposure to Accutane. Treatment of inflammatory bowel disease can involve surgery to remove part or all of the patient's colon.
Inflammatory bowel disease includes two different diseases: Crohn's disease and ulcerative colitis. Both affect the digestive system, although ulcerative colitis affects only the lining of the bowel, whereas inflammation from Crohn's disease can affect deep layers of the intestinal wall.
Patients with inflammatory bowel disease can develop serious problems including dehydration and anemia if the problem becomes severe. Problems associated with inflammatory bowel disease—such as diarrhea—can make it difficult for patients to continue on with their normal day-to-day living. Lawsuits have been filed against the maker of Accutane, alleging patients were not adequately warned about the risk of inflammatory bowel disease.
Accutane and Birth Defects
Shortly after international distribution, Accutane was in the news for allegations of causing severe birth defects when the mother took Accutane while pregnant. Birth defects included facial and bodily abnormalities and disorders of the brain, central nervous system, or cardiovascular system in the newborn baby.
In 2006, the FDA implemented the iPLEDGE program, designed to prevent the use of isotretinoin while pregnant. Patients who wanted to take Accutane were required to register for iPLEDGE, complete an informed consent form and, for women who were of childbearing age, comply with necessary pregnancy testing and be on two methods of birth control, unless the patient agreed to be abstinent.
Accutane and Suicide
 Before being removed from the market, Accutane was linked to an increased risk of depression, psychosis, suicidal ideation, suicide attempts and suicide. According to the FDA, from the time isotretinoin was marketed in 1982 through August 2004, there were 4,992 spontaneous reports of psychiatric disturbances associated with the use of isotretinoin in the US. Between 1982 and 2002 there were 165 reported suicides. However, the FDA noted that suicides linked to isotretinoin could be underreported, meaning there could be more than the 165 reported suicides. Prior to being removed from the market, Accutane the FDA warned that patients treated with isotretinoin should be observed for symptoms of depression or for suicidal thoughts.
Before being removed from the market, Accutane was linked to an increased risk of depression, psychosis, suicidal ideation, suicide attempts and suicide. According to the FDA, from the time isotretinoin was marketed in 1982 through August 2004, there were 4,992 spontaneous reports of psychiatric disturbances associated with the use of isotretinoin in the US. Between 1982 and 2002 there were 165 reported suicides. However, the FDA noted that suicides linked to isotretinoin could be underreported, meaning there could be more than the 165 reported suicides. Prior to being removed from the market, Accutane the FDA warned that patients treated with isotretinoin should be observed for symptoms of depression or for suicidal thoughts.
Accutane and Skin Reactions
In February, 2010, Health Canada and Hoffman-LaRoche issued a warning about the increased risk of serious skin reactions in patients who used Accutane. Those risks include Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Both conditions are rare but can be serious, resulting in hospitalization, disability or death.
Accutane's Manufacturer
Accutane Side Effects Legal Help
If you or a loved one has had adverse side effects from using the acne medication Accutane, you may qualify for damages or remedies that may be awarded in a possible class action lawsuit. Please click on the link below.Last updated on
ACCUTANE LEGAL ARTICLES AND INTERVIEWS
$25M Accutane Settlement Reinstated
Traded Clear Complexion for Depression and More
Accutane User Beyond Angry

January 26, 2017
Santa Clara, CA A $25.16 million award in favor of a man who alleges he developed Crohn’s disease as a result of taking Accutane has been reinstated by the New Jersey Supreme Court. The issue of alleged association between the acne drug and Crohn’s disease has been the subject of a decade long mass torte. Plaintiff Andrew McCarrell’s lawsuit is just one of 3,628 pending Accutane cases. READ MORE
Traded Clear Complexion for Depression and More

December 17, 2015
Passaic County, NJ: “I was a normal teenager before taking Accutane and my doctor assured me that it was safe,” says Jason. Many people like Jason, who suffer from Accutane side effects, no longer put their trust in doctors when they discover their doctors trust drug manufacturers. READ MORE
Accutane User Beyond Angry

September 2, 2015
Kelly stopped taking Accutane after just two weeks but she believes the acne medicine has already caused permanent damage. “Soon after I started Accutane I had serious abdominal pain and now I have been diagnosed with Irritable Bowel Syndrome - it’s not a stretch to connect the dots,” says Kelly. READ MORE
ACCUTANE SETTLEMENTS
- Accutane IBD Case Two Defendants Settle
- Accutane Lawsuit Results in $26 Million Jury Award
- Accutane Hoffman-La Roche to Pay 3 Teens Awarded $12.9 Million
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Suki
on
peter
on
Glenda
on
Baltimore, Maryland
on
Linda Colvin
on
EYESIGHT Side-Effects:
In 2002, I suffered a severe case of Dry Eyes (Keratoconjunctivitis Sicca), which caused permanent corneal damage to my left-eye, patches of elevated-scar-tissue (Salzmann's Nodules) to left-eye, contact lens intolerance, and caused some of the oil glands of both my eyes to become atrophied.
Currently, I'm scheduled for an upcoming eye surgery 08/11/16 to have the Salzmann's Nodules removed, however there's no guarantee as to whether or not the scars may redevelop. With my atrophied oil glands, correcting them would require a specialist squeezing the glands with a machine, which procedure costs up to $15,000 for both eyes. (Something my insurance will not cover).
INTESTINAL Side-Effects:
I've suffered off/on rectal bleeding over the years. Prior doctors dismissed it as anal fissures, but as of 02/08/16, I was officially diagnosed with a moderate to severe case of Crohn's Disease of both my small and large intestines with a fistula. I'm now required to take daily medication, and periodically need IV medication treatments, which procedures take 2 1/2 hours long to administer. These medicines are being used to help bring down my inflammation, and hopefully prevent me from requiring a future surgery down the road. These medicines carry their own risks, and lower my immune system and energy level.
I'm currently trying to find a product liability attorney who will help take my case.
Charles Chavez
on
I have not been diagnosed with IBS but it has Plagued me my whole adult life, I just live with it.
ivelisse Hernandez
on
Brian Sedlock
on
Brittany Clark
on
Kristina
on
Washington
on
British Columbia
on
Anonymous
on
North Carolina
on
Wisconsin
on
Washington
on
New York
on
Utah
on
Tennessee
on
Anonymous
on
Texas
on
California
on
Oklahoma
on
Manitoba
on
Texas
on
Alabama
on
Alberta
on
Ohio
on
Connecticut
on
Accutane was used for acne when he was 14 and 15.
Damages are Crohn's which he has had for 6 years. He is 23 now.
North Carolina
on
Oregon
on
New York
on